
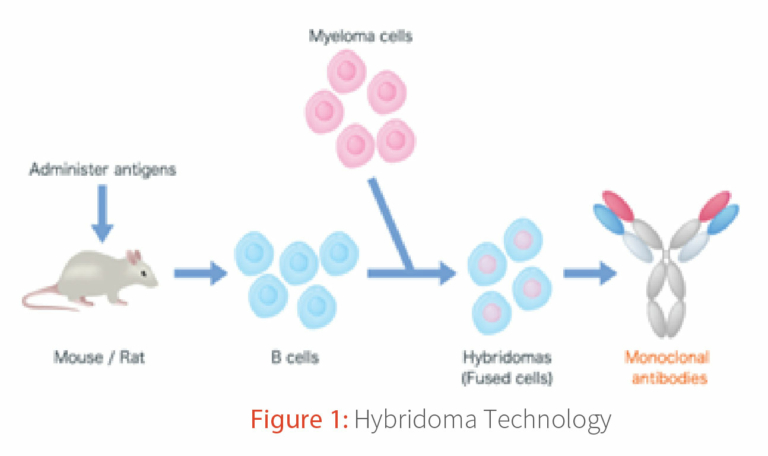
The standard hybridoma technique yields 90% of the antibodies authorized by the FDA1, 2. Monoclonal antibodies (mAb) have a broad range of applications, spanning diagnostics3, therapeutics4, and analytical tools5. Globally more than 80 monoclonal antibodies are approved and at present more than 700 antibody-based molecules are in different stages of clinical trials6.Through hybridoma technique (Figure 1) simple and efficient isolation of mAbs is possible and the method ensures native pairing of variable and constant region gene combination7. Additionally, the antibodies possess the natural pairing of variable heavy and light chain genes with naturally class-switched matured constant region gene through class switch recombination. Such spontaneous CSR flexibility is not attainable in other mAb isolation methods, making hybridoma a unique strategy for producing in vivo antibodies8.
Aragen life sciences has successfully established high-performing hybridoma cells producing platform for the successful production of range of mAbs. Aragen provides full-fledged services from antigen designing to high-throughput screening for binders against most native epitopes, including complex screens. In addition, we have a full capability for purification analytics and recombinant protein generation to cell line development.
At Aragen scientific personnel can expertly design and handle range of antigens. The production of effective specific antibodies is heavily reliant on successful and high-quality antigen design. A thorough selection, design, and synthesis of the most convenient antigen will allow the generation of superior antibodies suiting the diverse needs of clients from varied backgrounds. Aragen has capabilities to design different types of immunogens like, DNA, recombinant proteins, small peptide molecules, liposomes, and bead-based antigens. We have capabilities to design the antigen even when only In silico template is provided and we can also efficiently immunize with whole cell immunogen through cell line development process.
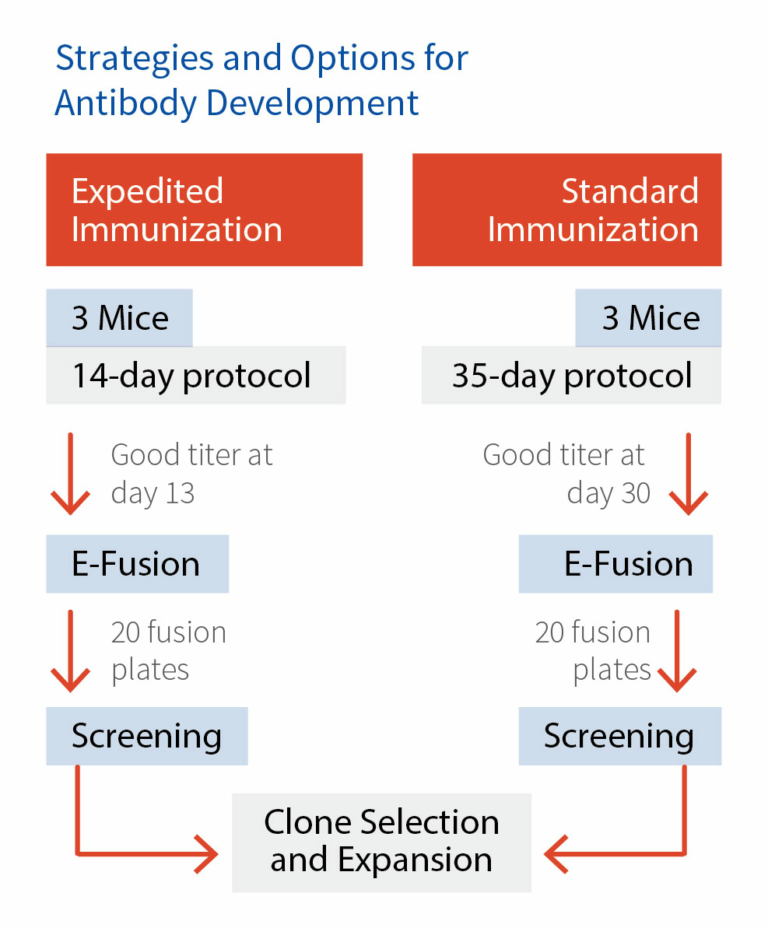
To meet the clients’ specific demands, Aragen customizes immunization methods. Aragen has inhouse 14-day protocol and a 35-day conventional protocol readily available and depending on the results obtained by both the methods we deliver the high-quality products. The optimization of both the protocols is carried out in two different strains of mouse and for each strain the experiments are performed in triplicate.
For immunization we target two different tissues of the mouse (Spleen and Lymph nodes).
In order to produce hybrid cells by the fusion of splenocytes with proper myeloma cells, Aragen has sophisticated fusion protocol dependent on high effciency electro-fusion system which has 20-50 times better effciency as compared to chemical (PEG) fusion methods.
Aragen performs high throughput screening using both the indirect and direct ELISA methods. Indirect ELISA is a defacto standard screening method in the hepten-specific antibody discovery. Direct ELISA is rarely used for routine hybridoma screenings because its sensitivity is generally lower compared to indirect ELISA, and individual high-affinity mAbs might be classified as a false negative. Aragen also performs high throughput FACS based screening. Compared with conventional screening methods FASC based screening technology is efficient enough to select the antibody-secreting or specific hybridoma in a time-saving manner.
The antibodies produced by hybridoma technique using normal mice models demonstrate varying degrees of immunogenicity and have many effector functions including, interactions with the complement system and naturally occurring Fc receptors on diverse blood white cells.
At Aragen the antibody production through hybridoma technology takes place through Alloy’s ATX-GK transgenic mouse, Harbour mice or wild type mice. Aragen is an Alloy and Harbour certified CRO. One of the most significant benefits of the transgenic mice method is that antibodies are produced using the same procedures discovered by César Milstein and Georges Köhler in 1975. The technology is well established, optimized, and understood, making the road to clinical trials and market approval simple.
Administration of biologics therapeutics can induce anti-drug antibodies (ADA), impacting their PK/PD characteristics. ADA may ultimately affect the efficacy and/or toxicity profile of therapeutic proteins. In cases when the ADA are produced in high amounts, they can even neutralize the drug’s therapeutic effects. It is critical to have access to precise PK/PD profiles of the mAbs generated while executing the hybridoma procedure.
Aragen considers these factors and develops a systematic strategy for assessing anti-drug antibodies (ADA) through different methods. This evaluation comprises a risk assessment as well as determinations of ADA content (concentration/titer), affinity, immunoglobulin isotype/subtype, etc.
Aragen has capabilities ensuring high quality humanized antibody production services under one roof. We deliver complete services from efficient immunogen design, high throughput screening platforms to ADA analysis of the biologics produced. Depending on the unique requirements, we provide global resources and excellent capabilities at every level of the hybridoma technology. To know more about our hybridoma services and to partner with us please contact us at bd@aragen.com.
