

Stable Cell Line Development (CLD) at Aragen – Accelerating speed to IND
 Cell line development
Cell line development
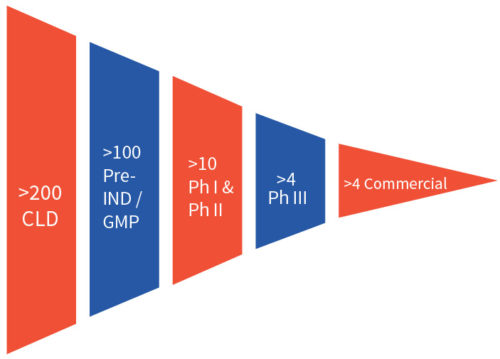
Efficient CLD is critical in bringing biologics to market and requires a team of experienced scientists, a portfolio of well validated cell line platforms, and ultramodern facilities. Aragen, a well-established preclinical CRO, delivers this combination and has completed more than 200 CLD projects, with over 100 of those cell lines in the clinic after successful investigational new drug (IND) filings. More than four of Aragen’s cell lines are producing marketed products.
Support for a Broad Range of Host Cell Lines and Expression Vectors
Aragen’s researchers have expertise in a wide range of host cell lines (CHO-DG44, CHO-GS, SP2/0, and NS0) and expression vectors (DHFR, Glutamine Synthetase (GS), and antibiotics). Aragen offers the royalty-free RapTr2022 platform, supporting CHO-DG44 and CHO-GS cell lines, which accelerates the cell line engineering process and offers higher titers.
Engineering protein solutions with robust analytical data.
20+ years experience in executing parallel processing and focused analytics to mitigate risks to client programs and shorten the timeline to IND, with scalable and reproducible processes.
