
Inflammatory pain is the perception and affective response to noxious stimuli that occur during an inflammatory or immune response. The inflammatory response involves coordinated physiological processes triggered by injury or infection and to reverse the pathology due to injury. Acute inflammation produces overt pain through the direct activation of sensory neurons that transmit the pain signals (Figure 1).
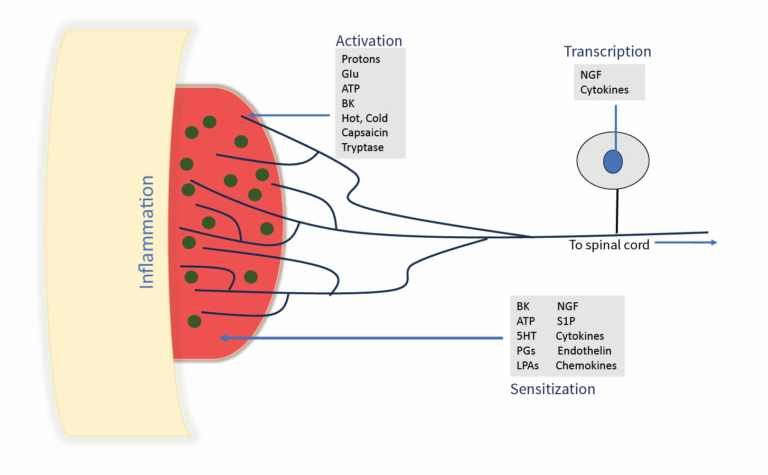
The onset of inflammatory pain is triggered by the activation of nociceptive sensory neurons via hot or cold thermal stimulation, mechanical stimulation, or inflammatory mediators. Followed by depolarization of sensory nerve endings in the peripheral region that initiate action potentials, which transmit noxious information through the spinal cord. (Glu:glutamate; ATP:adenosine triphosphate; BK:bradykinin; 5HT:5-hydroxytryptamine; PGs:prostaglandins; LPAs: lysophosphatidic acids;NGF:nerve growth factor; S1P:sphingosine 1-phosphate).
Clinically relevant preclinical animal models play a crucial role in elucidating inflammatory pain mechanisms and in turn assist in the development of effective therapies. Aragen Life Sciences offers a diverse array of preclinical pain models and treatment delivery routes. Our pain models have facilitated the preclinical screening of numerous promising candidates for our clients. This report is on the design and pharmacological validation of CFA -induced inflammatory pain model. Freund’s adjuvant includes of an antigen emulsified in mineral oil, serving as an immunopotentiator or booster. Freund’s Complete Adjuvant (FCA or CFA) consists of inactivated and dried mycobacteria, typically M. tuberculosis, while the incomplete form (FIA or IFA) lacks these mycobacterial components, representing a water-in-oil emulsion.
Aragen’s pain model is specifically designed to assess the anti-inflammatory properties of agents, such as non-steroidal anti-inflammatory drugs (NSAIDs), and the effectiveness of potential analgesic compounds in reversing cutaneous hypersensitivity. The studies using the CFA-induced rat model of inflammatory pain will be extremely helpful to screen compounds for their impact on heat hyperalgesia and tactile allodynia.
Our scientists have developed this model using male Sprague Dawley (SD) rats (280-300g). Inflammatory pain was initiated through a subcutaneous injection of 1 mg/ml of CFA in the ipsilateral hind paw. This led to prolonged swelling reaching its maximum at 24 hours and lasted for a minimum of 7 days. The inflammation was verified and quantified by assessing thermal/cold hypersensitivity in the ipsilateral hind paw.
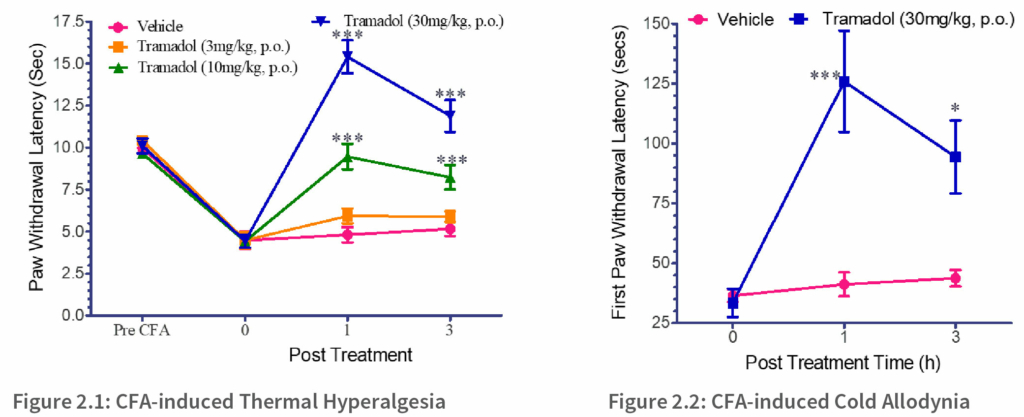
Thermal hyperalgesia and cold allodynia were confirmed by assessing paw withdrawal latency, which measures the duration of withdrawal (how long the limb stays off the glass plate). This will be an indicative of pain and hyperalgesia in inflamed animals. This is illustrated in Figure 2.1, where, before CFA induction, the animals exhibited a withdrawal response at approximately 10 seconds. However, following CFA treatment with the vehicle, the paw withdrawal latency decreased to less than 5 seconds. It was observed that tramadol exhibited a dose-dependent reduction in pain, peaking at 24 hours and gradually diminishing thereafter. Figure 2.2 illustrates the establishment of CFA-induced Cold Allodynia in rats. Like thermal hyperalgesia, tramadol e ectively reduced inflammatory pain for 1-hour post-treatment, with a subsequent reduction over time.
Aragen’s scientists have established an robust and highly effective CFA-induced inflammatory pain model. This model is often used to study the pathogenesis of inflammatory pain and the preclinical efficacy testing of the prospective therapeutic analgesic molecules. We offer customised protocols and flexible research protocols based on the client’s goals. Our highly skilled and experienced scientific team provides a reliable drug efficacy evaluation service supported with high-quality data in mutually agreed timeline.
To learn more about our pain models and In-vivo pharmacology services, write to us at bd@aragen.com.
