
Chronic pain is one of the main symptoms of diabetic neuropathy. Efficient pain medications are not available to sufficiently alleviate pain caused by diabetic neuropathy. Insulin deficiency is a primary cause of sensory neuropathy in type 1 diabetes. However, all the pathophysiological mechanisms for the development of chronic pain in diabetic patients is not fully understood. Nevertheless, it was established that the painful diabetic neuropathy is associated with hyperactivity of spinal dorsal horn neurons. Diabetic neuropathy is the term used to describe peripheral neuropathy in persons with prolonged history of diabetes. Simply put, diabetic neuropathy is caused by various types of nerve damages. Array of expanding research reveals that diabetic neuropathy is caused various pathological mechanisms such as uncontrolled protein kinase C activation, mitochondrial dysfunction, and reduced availability of different neurotrophic factors in sensory nerves. Early insulin therapy might normalize or prevent the development of chronic diabetic neuropathic pain in diabetic patients. Many researchers have investigated the impact of insulin therapy in rat models of diabetes induced by streptozotocin (STZ) and demonstrated that STZ alleviated diabetic neuropathic pain.
STZ is a naturally occurring alkylating antineoplastic agent (Chemotherapy) that affect the beta cells in the animal pancreas and deplete hormone insulin production. In medical research, STZ was used to develop animal models for type 2 diabetes or type 1 diabetes by administering several low doses.
Generally Male Sprague-Dawley rats (8 weeks old-220-260g) are used. Experimental diabetes was induced by intraperitoneal administration of 50 mg/kg of STZ. Rats exhibited pain hypersensitivity and reduced opioid analgesic effects, mimicking the clinical symptoms of diabetes. Diabetes was confirmed by measuring the glucose concentration in the tail-vein blood samples following three weeks of STZ injection.
Tactile allodynia is referred as mechanical allodynia, which is a condition where non-painful stimulus is perceived as painful. Innocuous touch sensory information from the skin is sent into the brain predominantly via the dorsal-column medial-lemniscus tract in the spinal cord. This sensory tract carries somatosensory information such as light touch. In tactile allodynia, these normally harmless sensory inputs may be perceived as painful.
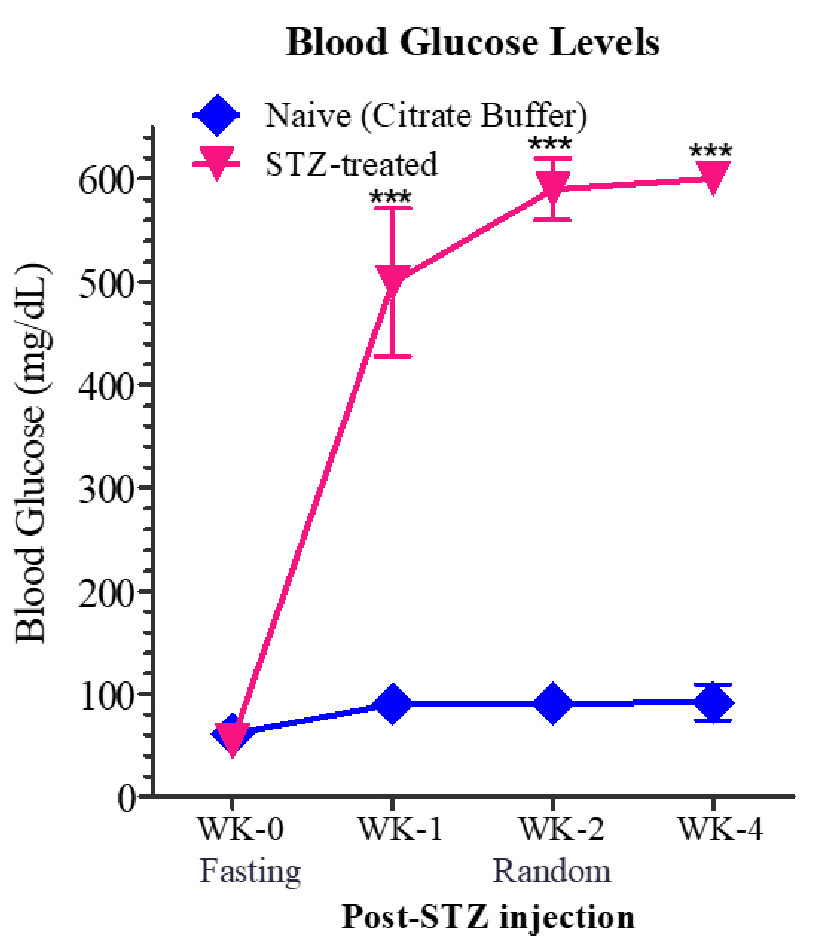
Hyperglycaemia was confirmed by measuring the glucose concentration in the tail-vein blood samples of control (injected with citrate buffer) and test animals. As seen in the Figure 1 the control animals have blood glucose levels below 100 mg/dL that was consistent for 4 weeks. However, the blood glucose level of STZ -treated animals was above 500mg/dL post 1 week of administration and increased to around 60mg/dL after 4th week of administration.
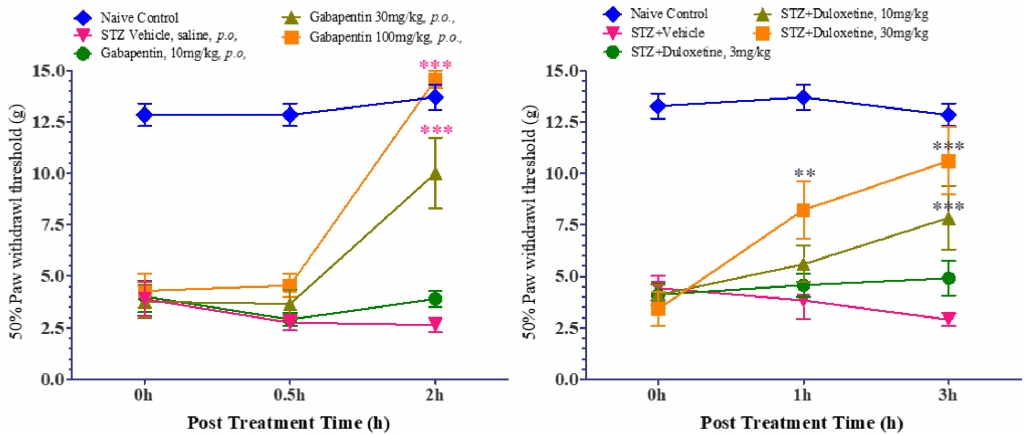
The tactile allodynia was validated by calculating 50% Paw withdrawal threshold following the application of mechanical stimuli with von Frey filaments. It was observed that that the control animals showed withdrawal response at ≥12g, whereas the STZ-induced rats showed withdrawal/pain response at lower force stimuli as indicated by the threshold values (50% Paw withdrawal threshold ≤ 5g). Gabapentin (anticonvulsant medication for neuropathic pain) and Duloxetine (FDA approved for the pain associated with diabetic peripheral neuropathy) exhibited dose-dependent significant reversal of tactile allodynia (Figure 2 and Figure 3).
Painful diabetic neuropathy is mainly associated with hyperactivity of spinal dorsal horn neurons although the precise underlying mechanisms are yet to be established. In addition, the pathophysiological mechanism leading to chronic pain development in diabetic patients is also not fully understood. The experts in vivo pharmacologists at Aragen have successfully developed a robust and reproducible model of STZ-induced Diabetic Peripheral Poly Neuropathic Pain. This model captures the clinical manifestations observed in humans with diabetic neuropathy.
Hence, we believe that this model is an appropriate model to screen new analgesics and to study pathophysiological mechanisms of diabetic peripheral neuropathic pain.
