
To evaluate the efficacy of XXX antibody (drug) (Test Article) product in comparison to Palivizumab, in controlling viral replication of RSV (Strain A2) in lungs of female cotton rats.
Thirty-five cotton rats (approximately 6-8 weeks old) were separated into 7 groups (N=5/ group).

On day -1, rats received a prophylactic intramuscular injection of test article at 4 mg/kg, 2 mg/kg, 1 mg/kg or 0.5 mg/kg or they received a prophylactic intramuscular injection of the control antibody, Synagis® at 4 mg/kg or 2 mg/kg. On Day 0, all animals were inoculated intranasally with 1×105 PFU of RSV strain A2. On day 4, serum, nose and lungs were collected following euthanasia and the viral lung titers were determined by plaque assay.
The test products and Synagis® exhibited dose-dependent antiviral activity in preventing RSV replication in the lungs of cotton rats infected with RSV A2. Treatment with test products (0.5 mg/kg, 1 mg/kg, 2 mg/kg or 4 mg/kg) significantly decreased viral lung titers compared to treatment with PBS (p<0.001). Furthermore, the test products decreased viral lung titers on average 16-fold more than the same dose of Synagis® (4 mg/kg group: p=0.021; 2mg/kg group: p<0.001).
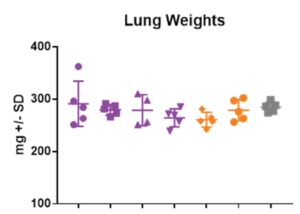
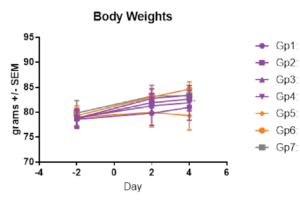
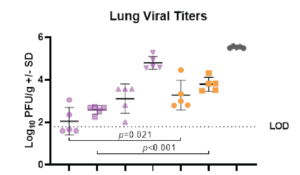
Aragen successfully completed preclinical investigations of the test compound on in-house female cotton rats. These challenge models are appropriate for preclinical studies of lead drugs developed for the treatment of respiratory disorders or for studying the pathophysiology of RSV infections.
Dr. Nagendra Ningaraj, Sr. Director – Scientific Affairs
Dr. Nagendra Ningaraj has done his Ph.D. from the National Institute of Mental Health and Neurosciences, Bengaluru, and postdoctoral research in Pharmacology and Toxicology from the University of Kansas Medical Centre. He has over 25 years of experience and has previously worked at Acuity Labs, Scintilla Bio-MARC Pvt. Ltd., Memorial Health University, Mercer University Medical School, Global Institute of Medical Sciences, Dr. Reddy’s Laboratories, and Vanderbilt University Medical Centre. He was a clinical investigator at PPD before joining Aragen. At Aragen, Nagendra leads the Product Management duties for biological services, with a focus on in vivo studies.
