
Investigations into pain resulting from surgical incisions are pivotal for the development of therapies targeting postoperative pain, a condition lacking effective and economical treatments. Bridging the gap between preclinical models of persistent pain (e.g., inflammatory, neuropathic) and acute postsurgical pain has prompted endeavors to establish methods for studying incision-induced pain. In this context, Brennan et al. introduced a rat model for postoperative pain in 1996 [1-2]. The model involves a plantar incision in the hind paw and is characterized by sustained, diminished withdrawal thresholds to mechanical stimuli, mirroring the conditions observed in patients post-surgery. The peak of pain behaviors occurs immediately after recovery from anesthesia, with heightened responsiveness persisting for several days before gradually diminishing. This postoperative pain model possesses unique attributes compared to other animal pain models: (1) it is incision-induced, (2) it is profound and enduring, (3) exhibits reduced withdrawal thresholds indicating mechanical hyperalgesia, and (4) the time course of pain behaviors mirrors characteristics noted in postoperative patients. Notably, this model demonstrates rapid onset of pain-related behaviors, with reduced withdrawal thresholds persisting throughout the testing period. It proves valuable for screening potential compounds effective in alleviating postoperative pain and enhancing our understanding of the pain mechanisms associated with surgical incisions.
Aragen’s in vivo pharmacologists have developed various clinically relevant preclinical models spanning diverse therapeutic areas. Our specialists have devised and validated numerous preclinical models for both neuropathic and inflammatory pain, employing surgical techniques or chemical induction methods. One such preclinical model focusing on incisional pain, developed at Aragen, has been employed for testing the efficacy of numerous innovative analgesic therapeutics developed by global pharmaceutical and biotechnology firms. Additionally, this model has been instrumental in exploring the etiology, sensitization, and plasticity associated with postoperative pain.
Surgical incision induces an acute pain because of severing of nerve endings which later modifies into an inflammatory
pain. The model mimics clinical surgery in humans with an incision to skin, fascia, and underlying muscle.
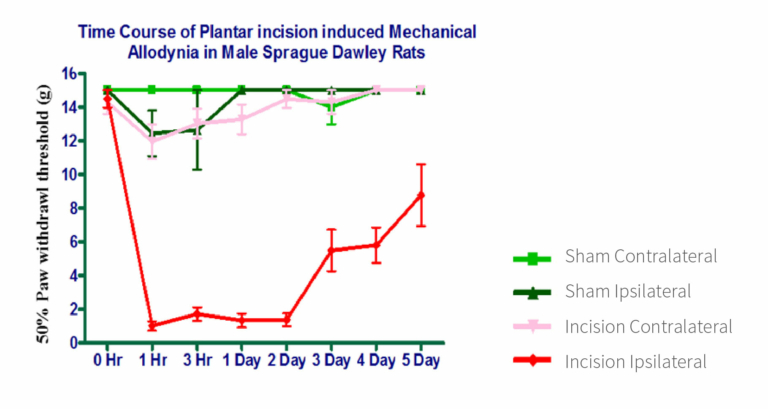
Mechanical allodynia in the operated animals was confirmed by determining the 50% paw withdrawal threshold after applying mechanical stimuli using von Frey filaments. The sham contralateral and sham ipsilateral groups exhibited a withdrawal response at ≥12g, which persisted for 5 days. Animals with incisions on the contralateral side also displayed a withdrawal response at ≥12g, although the values were lower compared to both sham groups (Figure 1). Within the incision ipsilateral group, the withdrawal response decreased to <1g, persisting until the second day post-surgery. Subsequently, it gradually increased until day 5 but consistently remained below 8g, signifying the effective establishment of mechanical allodynia in this particular group (Figure 1).
Researchers at Aragen Life Sciences have effectively developed a clinically relevant preclinical model for post-incisional pain. This model is robust and widely utilized for investigating the pathogenesis of post-operative pain and assessing the preclinical efficacy of potential therapeutic compounds.
We offer clients personalized protocols and flexible research methodologies tailored to their objectives. Our proficient and seasoned scientific team delivers a dependable drug efficacy evaluation service, providing high-quality data within the agreed-upon timeline. For further information on our pain models and in-vivo pharmacology services, please contact us at bd@aragen.com.
1.Brennan, T.J., Vandermeulen, E., and Gebhart, G.F. 1996. Characterization of a rat model of incisional pain. Pain
64:493-501.
2.Brennan, T.J. 1999. Postoperative models of nociception. ILAR Journal 40:129-136.
