
Prolonged QT intervals on an electrocardiogram, indicating delayed ventricular repolarization, pose a significant safety concern during the drug development phase. It is currently acknowledged that the primary cause of drug-induced QT prolongation often results from the direct pharmacological blocking of specific ion channels (such as the human ether-a-go-go-related gene (HERG) cardiac K+ channel) within cell membranes. Before advancing to clinical trials, it’s common to assess how a drug interacts with the HERG channel. This evaluation, aids in anticipating a drug’s impact on cardiac safety. The most precise and sensitive method for scrutinizing the biophysical and pharmacological aspects of the HERG cardiac potassium channel is through manual whole-cell patch-clamping.
The key objective of these studies is to investigate any blocking effect induced by novel drug candidates or new chemical entities (NCEs) on hERG channel stably expressed in range of mammalian cells. To this end, potassium current mediated by the hERG channel (called hERG current) is recorded at room temperature with conventional patch clamp technique using the whole cell configuration.
Despite its technical challenges, patch-clamp remains the key and reliable approach for exploring the intricacies of ionic channels. Manual patch-clamp is the benchmark for exploring the behavior of ion channels, applicable across a spectrum of cell types including cell lines, primary cultures, stem cell-derived cells, and tissue slices. Due to the flexibility of the technique virtually all ionic channels in the body: ligand-gated, voltage-gated, calcium-gated, fast/slow activating and deactivating – can be studied with unmatched resolution using well-designed electrophysiology protocols.
Aragen Life Sciences provides standard assays for investigating the impact of drugs on both voltage-gated and ligand-gated ion channels, along with personalized solutions and the development of customized assays.
Patch-clamp recordings involve the use of fine borosilicate electrodes with a tip diameter of 1-2 μm. A pipette filled with an electrolyte solution is securely attached to the neuronal membrane, isolating a specific portion of the membrane (known as a ‘patch’) electrically. The electrical currents passing through ion channels in this isolated patch are then directed into the pipette, where they can be precisely measured by an electrode connected to a highly sensitive differential amplifier. The tip of the pipette forms a high-resistance seal with the cell membrane, and by recording this current, valuable insights can be gained regarding the membrane’s conductance.
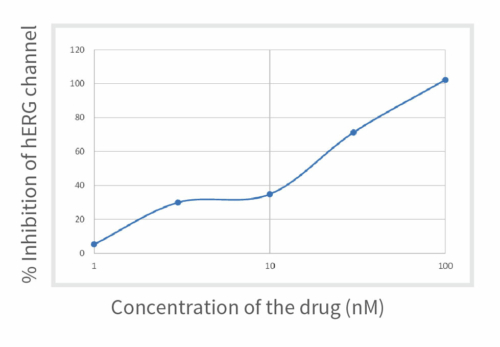
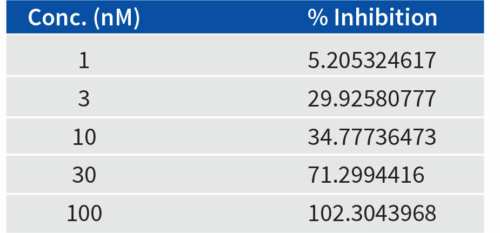
Aragen’s scientists meticulously performed manual patch clamp experiments to determine the potency of E-4031, an experimental class III antiarrhythmic medication that effectively obstructs hERG-type potassium channels. It was noticed that a progressive rise in drug concentration within the culture correspondingly resulted in a gradual escalation of hERG channel blockage, as evidenced by the % inhibition values. The outcomes are visually presented in Figure 1 and the values are in Table 1.
Aragen provides comprehensive electrophysical assay services. For detailed discussion with our subject experts on your specific requirements write to us at bd@aragen.com.
