
The International Association for the Study of Pain (2011) defines neuropathic pain as “pain caused by a lesion or disease of the somatosensory nervous system.” It can result from damage anywhere along the neuraxis: the peripheral nervous system, spinal cord, or supraspinal nervous system. Symptoms of chronic neuropathic pain include spontaneous pain, dysaesthesia, paraesthesia, allodynia, and hyperalgesia. Neuropathic pain management is very challenging due to its heterogeneity of aetiologies, symptoms, and complex mechanisms
Preclinical animal models are pivotal for understanding the mechanism of neuropathic pain and developing effective therapies for its optimal management. Aragen offers a wide range of neuropathic pain models and delivery routes that allow researchers to customize their studies. Our neuropathic pain models have been successfully used for preclinical screening of several promising candidates by our clients. We describe here the development and pharmacological validation of a Chronic Constriction Injury (CCI)-induced neuropathic pain model.
Chronic Constriction Injury was induced under gaseous anaesthesia. A blunt incision made on the skin overlaying the region between the gluteus and biceps femoris muscles, exposing the hind paw’s common sciatic nerve at the mid-thigh level. Approximately 7 mm of the sciatic nerve was released proximal to the sciatic trifurcation, and four (rats) or three (mice) loose ligatures of chromic guts were put around the sciatic nerve. Sutures in the muscle and skin staples were used to seal the wound. The animal was allowed to recuperate from surgery for 24 hours before pain hypersensitivity testing commences.
The surgery resulted in robust and consistent pain hypersensitivity for at least one month following injury. This sciatic nerve constriction was linked to intraneural edoema, localised ischemia, and wallerian degeneration. Mild to moderate autotomy, guarding, excessive licking, limping of the ipsilateral hind paw, and avoidance of placing weight on the injured side were noticed after the procedure.
CCI in rodents mimic clinical condition of chronic nerve compression that can occur after lumbar disk herniation or nerve entrapment in humans.
Tactile allodynia also referred to as mechanical allodynia, is a condition where non-painful stimulus is perceived as painful. Innocuous touch sensory information from the skin is sent into the brain predominantly via the dorsal-column medial-lemniscus tract in the spinal cord. This sensory tract carries somatosensory information such as light touch. In tactile allodynia, these normally harmless sensory inputs may be perceived as painful.
Chronic nerve compression or Nerve compression syndrome, or compression neuropathy, or nerve entrapment syndrome, is a medical condition caused by direct pressure on a nerve. It is known commonly as a trapped nerve, or nerve root compression by a herniated disc. The symptoms include pain, tingling, numbness, and muscle weakness and affect just one part of the body, depending on the nerve that is affected.
We treated the operated rat or mouse with the vehicle, Tramadol or Gabapentin to validate the tactile allodynia model. Tactile Allodynia was validated by measuring the mechanical sensitivity, quantified by measuring withdrawal response to punctuate mechanical stimuli applied with von Frey filaments. The injured animals treated with vehicle demonstrated the establishment of tactile allodynia as indicated by the paw withdrawal threshold values. The Tramadol and Gabapentin treated injured animals showed the reversal of the tactile allodynia in a dose dependent manner (Figure 1). Figure 2 represents the % maximum possible efficacy (%MPE) data. The log PWT value of the CCI-vehicle treated group was treated as base line and the log PWT values of the CCI-Tramadol groups were normalised accordingly to show the %MPE.
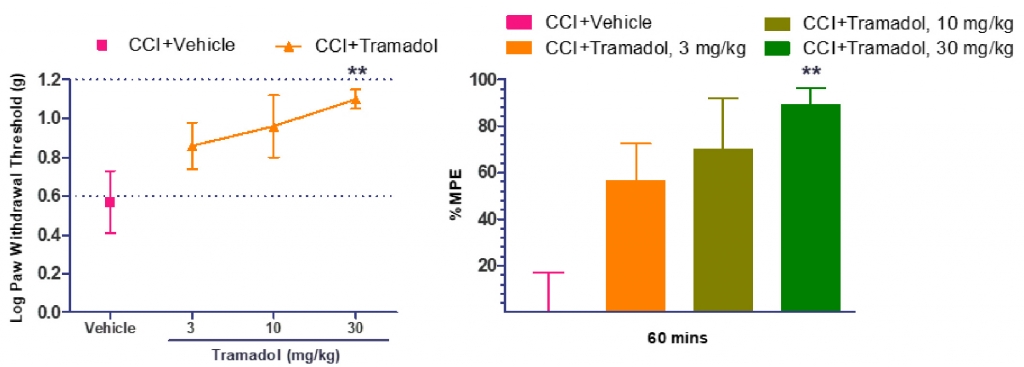
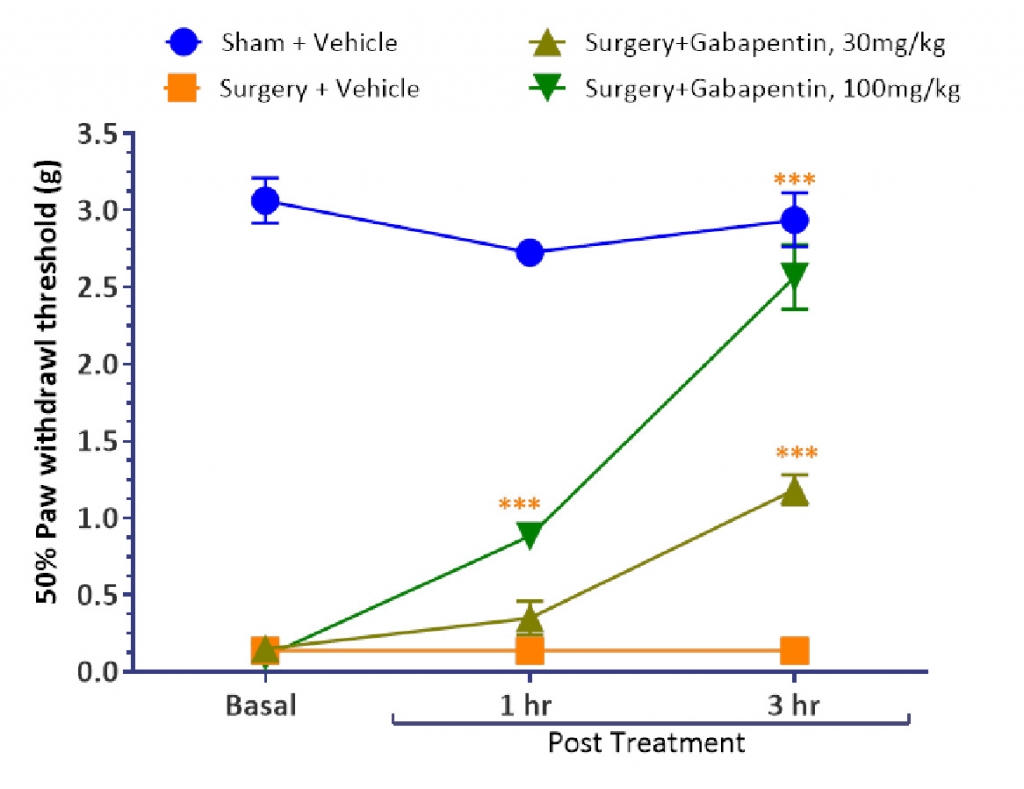
Figure 3 validates the establishment of Tactile Allodynia in CCI induced mice. It was observed that the Sham group of mice showed withdrawal response at ≥2.5g, whereas the mice with CCI were showing withdrawal/pain response at lower force stimuli as indicated by the threshold values (50% Paw withdrawal threshold ≤ 0.25g). The Gabapentin treated injured group showed the reversal of the tactile allodynia in a dose-dependent manner.
Scientists at Aragen life sciences have successfully established CCI-induced Neuropathic Pain model. This model is a robust and frequently used to study the pathogenesis of neuropathic pain and to evaluate the preclinical efficacy of the prospective therapeutic molecules.
We provide our clients with customised protocols and adaptable research methodologies based on their goals. Our highly skilled and experienced scientific team provides a reliable drug efficacy evaluation service with high-quality data within the mutually agreed timeline. To learn more about our pain models and In-vivo pharmacology services please write us at bd@aragen.com.
