

The pharmaceutical industry is increasingly challenged by the formulations of poorly water-soluble New Chemical Entities (NCEs), which often limits their oral bioavailability and, ultimately their clinical success. As molecular complexity rises, traditional formulation approaches often fall short in achieving optimal solubility and oral bioavailability.
Innovative drug delivery technologies are crucial for overcoming these solubility challenges, with, spray dried dispersions (SDDs) offering a scientifically validated and industrially scalable solution to enhance oral drug absorption.
CRDMOs (Contract Research, Development, and Manufacturing Organizations) play a pivotal role in bridging molecular pharmaceutics and scalable manufacturing. Leveraging specialized expertise, CRDMOs design, develop, and manufacture robust SDDs tailored for clinical and commercial success.
This white paper explores the molecular foundations and manufacturing science behind SDD technology, with focus on excipient selection, stabilization mechanisms, manufacturing science, and comprehensive development strategies to support effective drug delivery.
Approximately 70–80% of drugs in today’s discovery pipelines are classified as poorly soluble under the Biopharmaceutics Classification System (BCS Class IIa, IIb, and IV). Enhancing solubility and dissolution rates is often the critical gateway to realizing their therapeutic potential.
Among the available enabling technologies, amorphous solid dispersions (ASDs) produced via spray drying stand out for their unparalleled versatility, scalability, and clinical relevance. In Spray Dried Dispersions (SDDs), poorly soluble drugs are molecularly dispersed in a polymer matrix and converted to an amorphous form, significantly enhancing solubility, dissolution, and bioavailability.
CRDMOs bring the essential expertise in material science, pre-formulation, and molecular interactions—key to efficient SDD development. This white paper delves into the molecular pharmaceutics principles driving the design and optimization of high-performance spray dried dispersions.
Amorphous forms of pharmaceutical drugs are characterized by higher free energy relative to their crystalline counterparts, resulting in markedly enhanced apparent solubility and accelerated dissolution rates. This improvement is attributed to their disordered molecular structure, which facilitates more rapid dissolution than the tightly packed crystalline lattice, as depicted in Figure 1. However, the thermodynamic metastability of amorphous forms necessitates careful stabilization strategies to prevent recrystallization during processing, storage, and dissolution.
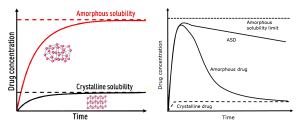
Spray drying of “as is” drug from a solvent facilitates rapid solvent removal, trapping the drug in an amorphous, high-energy state. Yet, these naïve amorphous systems are prone to devitrification to crystalline starting material, unless molecularly dispersed within a miscible polymer matrix, which markedly enhances both physical stability and dissolution performance.
Not all drugs exhibit equal ease of forming and maintaining an amorphous state.
Glass Forming Ability (GFA) is a critical factor in SDD success. Drugs with poor GFA require careful polymer selection to stabilize the amorphous form through:
Selecting polymers with good drug-polymer miscibility increase Tg, resulting in substantially reduced molecular mobility, slower crystallization kinetics, and higher physical stability during storage and in vivo dissolution.
Polymers are not merely inert carriers; they play an active molecular role in stabilizing amorphous forms, as shown in figure 2. Polymers such as PVP, PVP-VA, HPMC, and HPMCAS increase the Tg of the dispersion and additionally interact via hydrogen bonding, van der Waals forces, or ionic interactions to retard molecular mobility and inhibit crystallization of the amorphous pharmaceutical solids.
Another complimentary mechanism by which polymers stabilize the amorphous form is through favourable viscoelastic properties. Unlike stabilization via glass transition temperature (Tg) elevation, viscoelastic behavior offers a distinct kinetic advantage. During spray drying, certain polymers undergo a gelation transition before complete solvent
removal, creating a semi-solid matrix that significantly restricts molecular mobility—even when the temperature remains above the final Tg. This early viscoelastic “freezing” kinetically traps the amorphous state, minimizing recrystallization risks during particle formation.
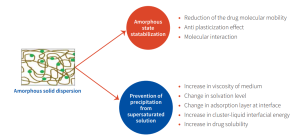
Leading CRDMOs routinely perform developability assessments to characterize a drug’s glass forming behaviour—whether strong or fragile—and select polymers that provide one or both Tg and viscoelastic advantages, thereby maximizing solubility benefits while ensuring stability.
Miscibility between the active pharmaceutical ingredient (API) and polymer is critical for achieving a homogeneous molecular dispersion. A truly miscible system eliminates phase-separated domains that could act as nucleation sites for crystallization. In contrast, poor miscibility leads to localized enrichment zones with higher molecular mobility, significantly increasing crystallization risks. Different systems of homogeneous, heterogeneous and nucleation and
crystal growth are show in figure 3. Therefore, enhancing drug-polymer miscibility is a fundamental strategy to control molecular mobility and maintain amorphous stability at the nanoscale.
Key factors influencing miscibility:
Water and humidity pose significant challenges to the stability of amorphous dispersions. Moisture acts as a potent plasticizer due to the extremely low glass transition temperature (Tg) of water (–135°C). Even small amounts can lower the overall Tg of the system, increase molecular mobility, and ultimately compromise the stability and solubility advantages of the amorphous form.
CRDMOs design SDDs to minimize moisture sensitivity through:
Bridging molecular design to commercial-scale manufacturing demands precise control over multiple parameters:
Advanced spray dryers equipped with closed-loop nitrogen systems and in-line monitoring technologies ensure robust process control and compliance with cGMP standards at leading CRDMOs. Through rational polymer selection, meticulous miscibility screening, and precision spray drying CRDMOs can accelerate development timelines while minimizing risks in clinical drug product development based on SDD technologies.
Spray dried dispersions represent a mature yet continually evolving technology at the intersection of material science, molecular pharmaceutics, and manufacturing excellence. Maximizing their potential for poorly soluble drug candidates requires a deep understanding of key SDD developability considerations like polymer selection, drug-polymer interactions, molecular mobility, viscoelastic behavior, and robust process design. Strategic application of these principles enables the development of stable, bioavailable formulations that accelerate clinical timelines and support commercial success.
At Aragen Life Sciences, we offer end-to-end SDD solutions, combining deep scientific expertise with advanced manufacturing capabilities. Our integrated approach ensures optimal formulation design, scalable process development, and reliable cGMP-compliant production. By partnering with Aragen, clients can unlock the therapeutic potential of even the most challenging drug candidates, accelerating their path from discovery to market.
