 Back to News
Back to NewsBest practices & strategies for biotherapeutics production using a stable scale-up processDr Anurag Dhobal and Dr Nagendra Ningaraj of Aragen Lifesciences look at the challenges and options available to improve biologics production*
March 23, 2023

Biopharmaceutical medicinal products, or biologics, are already an integral component of therapeutic strategies to treat various human diseases.1 Biologics, which mostly consist of therapeutic recombinant proteins, are used in treating a variety of diseases, including malignancies and inflammatory disorders.2
Developing a dependable commercial biopharmaceutical manufacturing method is logistically and technically complex, with several potential hurdles. Small biotech and major pharma businesses are constantly seeking partners to expedite the production of medications with a shorter timeline and higher yield and quality.
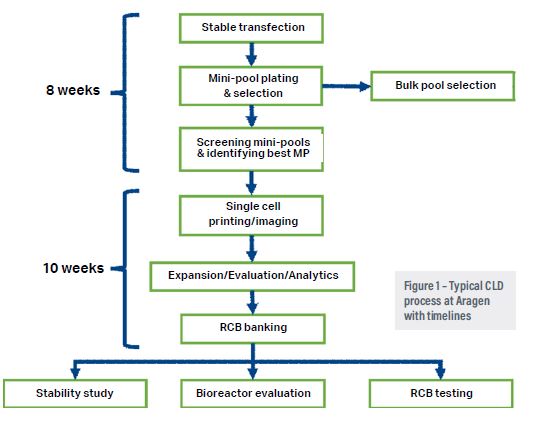
The main challenge with the accelerated discovery and production of biologics is exclusive dependence on live host cells that act as biological factories. Cells are sometimes unpredictable and might present technical problems.3 Thus a robust, high-yielding cell line platform is the basis of a dependable manufacturing process.
For instance, biologic instability might occur during the development phase or during the scale-up process itself. A small change in pH or temperature, or a change in the consumables can impact the anticipated structure and function of the biologic.4 Advance, precise prediction of the cell culture conditions on a biologic’s function can lead to effective process scale-up and greatly decrease regulatory time delays.
Many CROs face difficulties in scaling up biologics production due to inefficient analytical facilities, unstable molecules and limited process development capabilities. All these factors influence the time to market and budget outcomes of the biologic’s developers.
A comprehensive analytical facility requires huge capital investment, which ultimately depends on the organisation’s operations and commercial size. Hence, understanding and alleviating the potential barriers to efficient production and scale-up procedures before starting the project is critical for the project’s success.
In this article, the challenges associated with the biologics production process (cell line development, CLD) are discussed briefly, followed by strategies to avoid these challenges, which will assist biopharma companies in de- risking their production and scale-up methodologies from the drawbacks of misinformed decision-making.
Key challenges in production
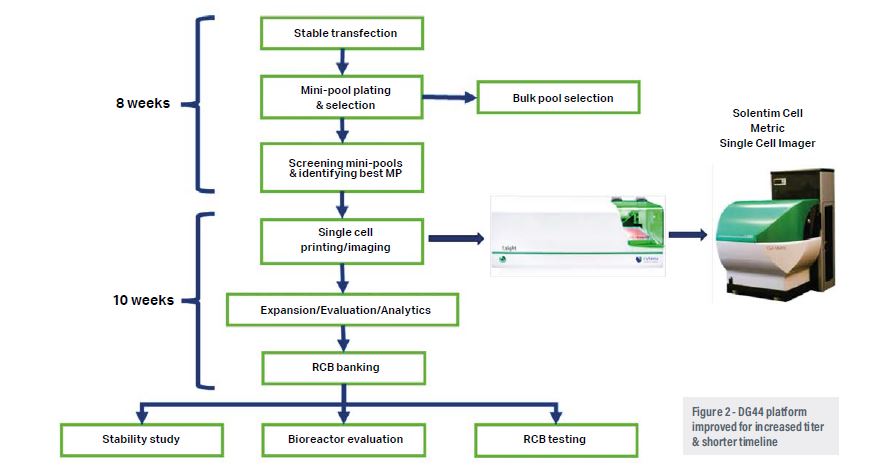 Selecting the right expression platform and vector for process development at pilot-scale and commercial manufacturing is a major challenge. Biologics made up of several monomeric components have complicated structures, mechanisms of expression, post-translational modifications and transport within the cell to be functionally active.5
Selecting the right expression platform and vector for process development at pilot-scale and commercial manufacturing is a major challenge. Biologics made up of several monomeric components have complicated structures, mechanisms of expression, post-translational modifications and transport within the cell to be functionally active.5
A one-size-fits-all approach is unsuitable. The appropriate platform should express the molecule in its fully functional form, and be simple to handle (transfection) and scalable. Some elements for CLD success include a traceable and thoroughly recorded history, legal needs, growth characteristics and cell culture conditions.
Identifying a better performing clone is difficult and time-consuming. Screening procedures require substantial time, considerable optimisation of many assays and testing using purified chemicals. Expert professionals will be involved in clone selection and checking its performance at various bioreactor levels.
There are many challenges in analytical characterisation. The chosen clone must be amplified and thoroughly characterised. To ensure safety and efficacy, orthogonal characterisation methodologies should be used.6 Comprehensive characterisation of biologics is feasible using a variety of assays, each of which is required in order to obtain information about the product’s physicochemical qualities. Performing these assays demands the use of modern equipment and established protocols.
Establishing a high throughput analytical laboratory is costly. Handling this equipment and carrying out the procedures necessitates experienced scientific staff with competence across numerous disciplines, which is challenging for small businesses to procure. Furthermore, analytical characterisation is prone to manual errors, which must be corrected by qualified technicians.
There are also challenges in process development to address. Commercial-scale biologic production is only possible when bench-scale prototypes are scalable to pilot- scale and high-volume bioreactors.7 Parameters should be established both at the shaker flask scale and at the larger-scale reactors to avoid wasting valuable resources and time. Therefore, it is critical to establish the production parameters following rigorous experimental design and the verification of as many variables as feasible. Process development must also include parameter testing at various bioreactor volumes, which is expensive and can cause considerable delays.
Besides technological problems, biologics manufacturing has several logistical challenges. To mitigate these challenges, a client needs to make sure that a CRO will be able to complete the entire process in-house from beginning to end. In addition, a GMP-compliant facility and a committed scientific workforce with robust project management skills are essential.
One way to reduce time-to-market is to outsource production to established CROs and CDMOs that specialise in large molecules and have the appropriate competence. Another, which requires significant capex, is to develop in-house capabilities, such as investing in some of the most modern technology and characterisation services in commercial biologics manufacturing.
Strategies for biologics production
We have identified some of the strategies that can help firstly to accelerate the biologics production and scale-up processes and, secondly, to overcome the challenges associated with it.
Using established host cell lines for biopharmaceutical expression can significantly shorten manufacturing procedures and minimise risks in development. Established platforms are scalable, can accommodate a wide range of compounds, including difficult-to-express proteins, and have their own completely defined media systems.
They are intended to eliminate unpredictability, maximise yields and shorten development timelines dramatically. Furthermore, established cell lines will have a complete history and a track record of successful projects.
To accelerate downstream process development, established and proven expression vectors must be used when engineering novel cell lines. Efficient vector systems give a steady expression of the inserted genetic material without exhibiting any side effects on host cells.
Testing multiple platforms in parallel is another key strategy, especially when clients are unsure about the best host cell line platform to use. With many alternatives available, determining which platform would work best for a particular product is very difficult.
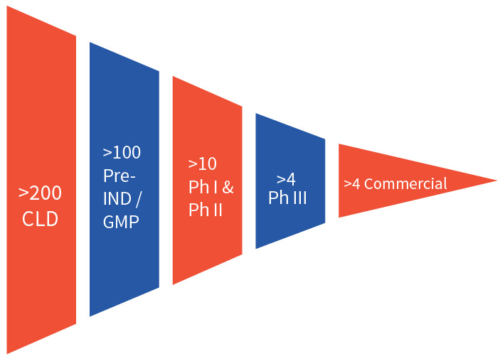
Therefore, many clients who understand the diversity of biologics and the unpredictability of biological systems choose the parallel testing of multiple platforms at the start of a programme. This strategy helps developers to mitigate the risk of failure and increase the odds of success in the very early stage of biologics development.8
A comprehensive plan must be devised that describes the need and rationale for post-transfection investigations and assays. The protocols that will affect biologics from start to finish, including experiments and methodologies for small-scale characterisation and full-scale validation, must be included in the plan.
The plan must outline the efforts that will be made to execute and finish the different activities in accordance with cGMP. It must also guarantee that a well thought-out, justified and thorough process characterisation is achieved.
The risks associated with various stages of process development (such as minipool characterisation, single cell cloning, screening, etc.) must be rigorously evaluated at bench- scale, since they can affect process features and product quality attributes. Critical product characteristics should be examined during analytical characterisation, depending on the risks recognised.
Using modern and high-throughput characterisation tools is critical for carrying out large screening operations and eliminating the possibility of human mistakes. Such tools help reduce both optimisation time and the use of reagents and consumables. The primary benefit of advanced technologies is that they are based on quality by design principles, and so are ideal for scaling and identifying hazards associated with the later phases of process development.
Advanced characterisation tools ensure that the testing methodologies are reliable. These procedures are critical for the analysis and characterisation of biologics, as well as guaranteeing the product’s safety, stability and efficacy at all stages of development.9 These technologies are also essential for rapid clonality assurance to shorten cycle times for FDA submissions.
A second round of risk evaluations on the process parameters should be undertaken based on the analytical characterisation data. As a result, a list of critical and non-critical parameters is generated, as well as a prioritisation of the critical process parameters. Automation allows for remote monitoring as well as shift production to maintain a socially separated safe atmosphere. It enables continuous production around the clock with less in-person supervision, as well as enhanced uniformity to aid in the conservation of critical components like adjuvants.
Automation and digitisation also enable the collection of massive volumes of quality assurance data to create a comprehensive audit trail and develop predictive modelling initiatives.10
Outsourcing cell line development packages is an important option. Before beginning a project, developers should carefully assess their capabilities for small-, pilot- and commercial-scale, GMP-compliant production. Where they are unsure, outsourcing the whole project or the most time-consuming or operationally difficult elements to well-established CROs with extensive domain experience might be the best alternative.
Established vendors who specialise in large molecule production and scale-up have experience of working with a wide range of molecules and thus understand the risks involved, as well as possible solutions. They also have all the modern technologies and expert manpower in place as well as solutions to a wide range of logistical and regulatory challenges.
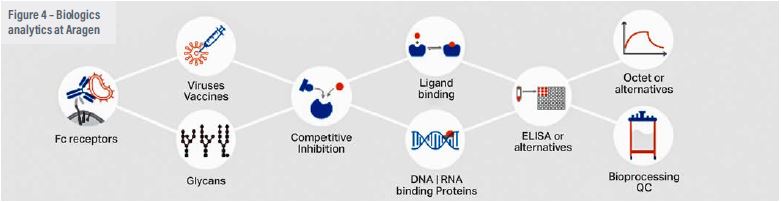
Raw material quality should be one of the primary considerations for biologics manufacturing because impure reagents and consumables used in the initial phases of production might pose significant problems in the scale-up process. It will have an influence on performance metrics (titres, PVCD) and cause batch-to- batch variations.
The primary goal of scale-up is to enhance yield while preserving functional quality. The contaminants in the media affect post-translational modifications, such as glycosylation. Trace element analysis and adding supplements such as peptones, animal origin-free and/or chemically defined components can all be used to improve performance or maintain productivity at higher volumes.
Analysis of the cost of goods sold (COGS) associated with the scale-up process is critical. COGS fluctuates with the physiochemical features and scale of the biologics, so analysing it helps in making crucial choices, such as modifying process protocols or discontinuing development.
Efforts must be made to develop a scale-up procedure that is both cost- effective and efficient. Single-use technologies, perfusion processes instead of fed-batch operations, continuous chromatography and end- to-end continuous processes can all reduce COGS in bioprocessing.
Early optimisation of the formulations can likewise reduce the number of unsuccessful batches and the amount wasted material associated with process development. Process changes like moving from liquid to powder media to save on shipping expenses or switching to a perfusion procedure to boost bioreactor efficiency are another way.
The basic requirement for regulatory acceptance is the correct traceability of each component used in the formulation. Using approved and traceable reagents and consumables, delivered with full regulatory documentation, along with thorough product characterisation, helps to smooth the approval process.
Clinical trials demonstrating that the product is safe, effective and reproducible are an essential requirement of the FDA. cGMP regulations require all commercially produced biologics to meet stringent assay, quality and purity requirements. Regulations differ from country to country but all require a well- optimised product and a cost-effective, reproducible scale-up process.
Conclusion
The production and scale-up of biologics requires well-informed decision-making. There are numerous challenges and the upstream process development and scale-up methodologies come with various risks. Developers seek to design robust, reliable and reproducible protocols for cost-effectiveness and quick market access but the timelines and expenses budgeted are often idealistic. To fast-track process optimisation, the correct decisions must be made early, which requires rapid access to high-quality data that can only be generated with established production platforms and advanced characterisation packages. Adopting modern and automated technologies from the inception of the project is necessary for identifying critical and non-critical factors influencing rapid, cost-effective market placement. The large molecule industry is under extreme pressure, due to a huge number of competitors, limited budgets and tight timelines. One way to ensure successful delivery is to partner with an established vendor that has completed many projects and has experience with a range of biomolecules, including difficult-to-express proteins. Ultimately, taking informed decisions is critical to the successful production and scale-up of biologics, as well as the seamless delivery of the new therapy to the clinics.
* – The authors wish to acknowledge Drs Sufia Karim and Divya Khaitan of Aragen’s Department of Biologics for allowing the schematics to be used in this article and the formatting effort of the corporate communications team in India
References:
1: W. Wang & M. Singh, eds., Biological drug products: Development & strategies, 2013
2: R.M. Lu, Y.C. Hwang, I.J. Liu, C.C. Lee, H.Z. Tsai, H.J. Li & H.C. Wu, J. Biomed. Sci. 2020, 27(1), 1-30
3: S. Fischer, R. Handrick & K. Otte, Biotech. Adv. 2010 33(8), 1878-1896
4: X.Y. Li, M. Cheng, J. Li, X. Zhao, Y.S. Qin, D. Chen, J.M. Wang, & C.F. Wang, J. Dairy Sci. 2020, 103(2), 1337-1351
5: J.M. Marchingo & D.A. Cantrell, Cell. & Mol. Immunol. 2022, 19(3), 303-315
6: N. Nupur, S. Joshi, D. Gulliarme A.S. Rathore. Front. Bioeng. Biotec. 2022, 10, p.36.
7: Commercial-Scale Biopharmaceutical Production – BioProcess InternationalBioProcess International (bioprocessintl.com)
8: Testing Cell Line Development Technology
Platforms In Parallel (outsourcedpharma.com)
9: Confirming Protein Identity and Purity | BioPharmaSpec
10: Bioprocessing 4.0 – Where Are We with Smart Manufacturing in 2020? | Pharmaceutical Outsourcing – Journal of Pharmaceutical & Biopharmaceutical Contract Services (pharmoutsourcing.com)
Source – SPECIALITY CHEMICALS MAGAZINE
