
Reporter cell lines are genetically engineered cells that express reporter genes, allowing monitoring of cellular processes through detectable signals like luminescence or fluorescence. In immuno-oncology, these cell lines are useful for monitoring T-cell activation, a critical functional readout for assessing immune responses to therapies. By linking reporter gene expression to pathways like the NFAT (Nuclear Factor of Activated T cells) pathway, these systems enable precise, quantifiable measurements of T-cell activation, critical for evaluating immunotherapies, offering valuable insights into the effectiveness of immunotherapies.
This case study details the development of a Jurkat-NFAT-Luc reporter cell line, optimized for sensitive, reproducible detection of T-cell activation. By incorporating an NFAT-responsive luciferase construct into the Jurkat T-cell background, this system offers a reliable platform for assessing immune-modulating agents, supporting early-stage evaluation and high-throughput screening.
We generated NFAT-Luc reporter Jurkat cells by introducing a plasmid carrying the luciferase gene under the NFAT response element via electroporation. Following antibiotic selection, stable Jurkat-NFAT-Luc cells were established. The functionality of these cells was validated by stimulating them with T-cell activators or chemical stimulants, to confirm stable integration and activation of luciferase expression through both TCR-dependent and independent signaling pathways.
To evaluate the responsiveness of the Jurkat-NFAT-Luc reporter cell line to T-cell stimulation, a 96-well plate-based functional assay was performed that involved:
1. TCR-Dependent Stimulation Using Anti-CD3/CD28 Beads
2. TCR-Independent Stimulation Using PMA and Ionomycin
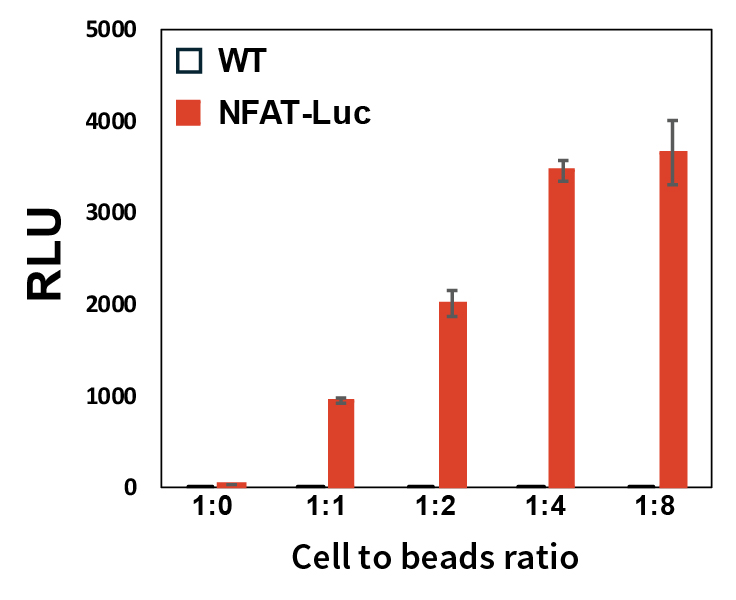
Figure 1: Response of Jurkat-NFAT-Luc and Jurkat wild-type (WT) cells to anti- CD3/CD28 beads. Error bars represent ±SE.
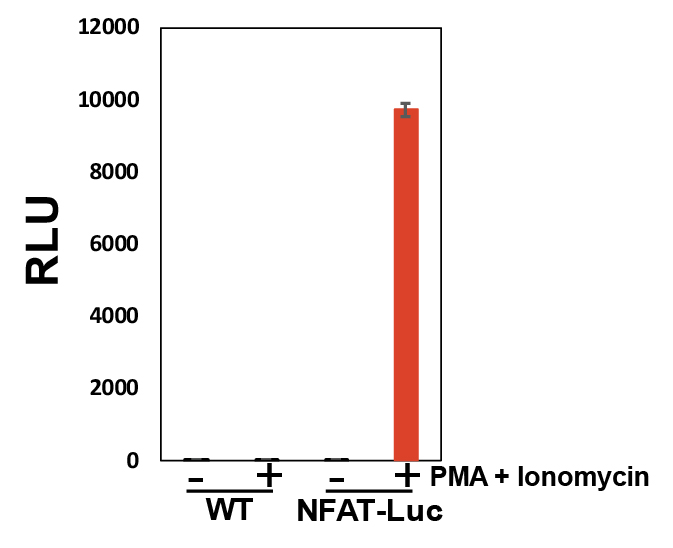
Figure 2: Response of Jurkat-NFAT-Luc cell line upon treatment with a combination of PMA and ionomycin. Error bars represent ±SE.
These findings confirm the Jurkat-NFAT-Luc cell line as a reliable tool for tracking T-cell activation, both through TCR-dependent and TCR-independent pathways, supporting its use in evaluating immuno-oncology therapeutics.
At Aragen, we specialize in custom cell line development for drug discovery, including immuno-oncology applications.
We offer:
Accelerate your research with our reliable, high-quality cell line development. Visit our website and get in touch to learn more!
