Racemization involves transforming an optically active compound into its racemic (optically inactive) counterpart through either heat or a chemical reaction. This results in an equal 1:1 ratio of enantiomers, forming what is known as a racemic mixture. Typically, reactions are specific to one stereoisomer, leading to the desired product, while the other stereoisomer remains unreactive or might trigger undesired side effects.
This case study presents a project executed by Aragen’s experts in process development and scale-up. The project’s aim was to carry out the racemization of an undesired compound. This compound was synthesized by the client for treating inflammatory conditions. The client encountered a challenge as they couldn’t successfully generate the racemic mixture of the compound during batch racemization processes, and the compound’s purity was declining. Given Aragen’s expertise in racemization, they were approached to help achieve the biologically active racemic mixture while maintaining the compound’s purity
To perform racemization of an unwanted compound to achieve a biologically active racemic mixture with high purity. The project involved two stages: initial experimentation in a batch system to identify and understand the challenges faced, and subsequent implementation in a flow system. The transition to the flow system served a dual purpose – addressing challenges encountered in the batch setup and enabling the scalability of the process for large-scale production of the compound while maintaining the purity.
The client is a biotechnology company headquartered in the United States, specializing in drug research and development especially focusing on creating innovative small-molecule therapies for immune-inflammatory conditions.
Batch experiments: Undesired compound was taken in 15 vol. of toluene and heated to reflux for 5-6 hrs.
Issue in batch racemization: Due to longer exposure under reflux conditions purity of compound decreased from 99.7% to 92% as indicated by HPLC analysis.
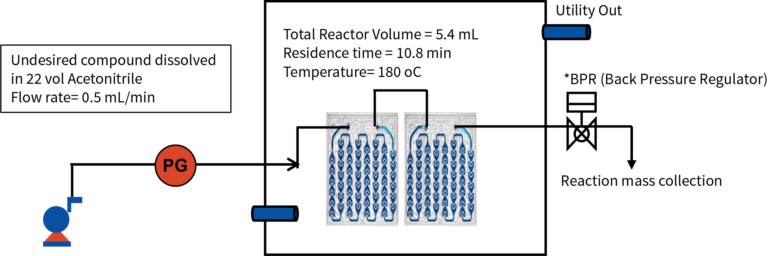
The undesired compound was dissolved in 22vol of Acetonitrile (ACN) at 25-30°C and was passed through Corning flow reactor at 180°C under back pressure of 11 Bar. (schematic representation: Figure 1)
Residence time: 5.4 min
Flow rate: 0.5 mL/min