
Models help in comprehending the underlying mechanisms and pathophysiology of neuropathic pain. They aid researchers in studying the processes that lead to nerve damage or malfunction, which in turn cause neuropathic pain.
These models serve as a basis for testing and developing new drugs or treatments for neuropathic pain. They allow researchers to assess the efficacy and safety of potential medications before clinical trials in humans.
Models aid in evaluating the effectiveness of existing treatments or therapeutic interventions for neuropathic pain. This helps researchers and clinicians understand how well current therapies work and if there are any limitations or side effects.
They provide insights into the pathways and neural circuits involved in transmitting and processing pain signals in neuropathic conditions. This knowledge is essential for devising targeted therapies that can specifically modulate these pathways.
The model is developed using Male SD Rats weighing 230 – 250 g. Neuropathic pain and whole-body hypersensitivity is developed by administrating multiple doses of paclitaxel at 1mg/kg through intraperitoneal route.
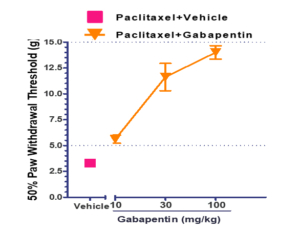
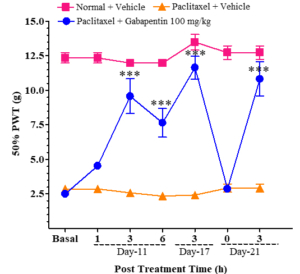
The sensitivity of the hind paw in treated animals to touch and temperature changes was confirmed through tests that measured the 50% paw withdrawal threshold (PWD) following the application of mechanical stimuli with von Frey filaments. Animals treated with paclitaxel exhibited withdrawal responses at ≤ 5g, while those treated with both paclitaxel and gabapentin showed 50% PWD values ≥ 5g. As the concentration of gabapentin increased, there was a gradual rise in PWD values (see Figure 1). Figure 2 displays the post-treatment time for the 50% PWD across different animal groups. Gabapentin, a medication primarily utilized for neuropathic pain (nerve pain), falls under the class of anticonvulsants or antiepileptic drugs. Its mechanism involves influencing specific chemicals and nerves in the body that contribute to seizures and various types of pain.
The experts in vivo pharmacologists at Aragen have successfully developed a robust and reproducible model of paclitaxel-induced poly neuropathic pain. The model is robust and captures the clinical manifestations observed in humans with poly neuropathy. The model is an appropriate tool to screen new analgesics and to study pathophysiological mechanisms of poly neuropathic pain.
