
Experimental pain models that mimic key aspects of human pain ethology are essential in understanding the pathophysiology of pain mechanisms and test new pain medications under controlled preclinical settings.
Management of pain in clinics remains a significant global health problem, despite intense research efforts of leading pharmaceutical companies in developing a potential analgesic- for more than 2 to 3 decades no novel analgesics has been discovered. Studies conducted using intact animals allow the examination of the multidimensional nature of pain. Aragen scientists have developed various animal models to evaluate the efficacy of novel analgesic drug candidates against inflammatory, neuropathic and cancer pain. We describe here the development and pharmacological validation of Spinal Nerve Ligation (SNL) model of Neuropathic Pain.
The SNL model of neuropathic pain is widely used for investigating mechanism of neuropathic pain and to screen potential analgesics. This rat model was developed by ligating lumbar nerves of the spinal cord. The surgery resulted in long-lasting pain like behavioural sign as revealed by mechanical allodynia.
The SNL model showed robust sign of mechanical allodynia, and hence can be used the measure the degree of hypersensitivity of the paw. Mechanical sensitivity is quantified by measuring withdrawal response to punctuate
mechanical stimuli applied with von Frey filaments. This is a technique commonly used in clinics to test touch sensation in humans.
SNL rodent model is comparable with the clinical condition of nerve injury to nerve plexus or dorsal
root in humans.
Tactile allodynia also referred to as mechanical allodynia, is a condition where non-painful stimulus is perceived as painful. Innocuous touch sensory information from the skin is sent into the brain predominantly via the
dorsal-column medial-lemniscus tract in the spinal cord. This sensory tract carries somatosensory information such as light touch. In tactile allodynia, these normally harmless sensory inputs may be perceived as painful.
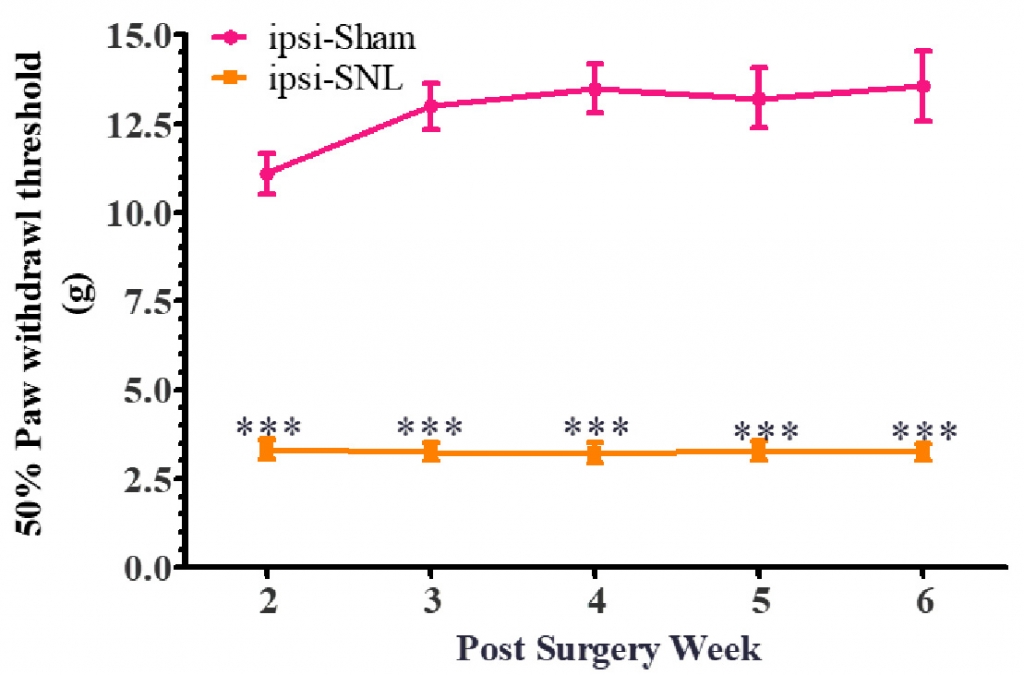
The tactile allodynia was validated by calculating 50% Paw withdrawal threshold following the application of mechanical stimuli with von Frey filaments. It was observed that that the Sham group of rats showed withdrawal response at ≥12g, whereas the rats with SNL were showing withdrawal/pain response at lower force stimuli as indicated by the threshold values (50% Paw withdrawal threshold ≤ 4g) (Figure 1). The measurements were done for 6 weeks post-surgery, and the tactile allodynia response lasted up to 6 weeks and results were consistent across the testing period.
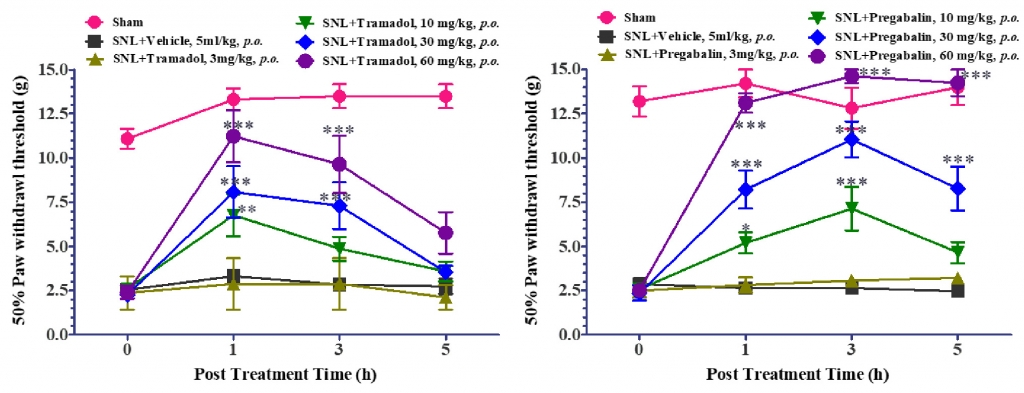
To further validate the effectiveness of the model, SNL rats were randomly divided into vehicle and treatment groups treated with Pregabalin and Tramadol. Sham and vehicle SNL rats were treated with specific vehicle via oral route. Tramadol and Pregabalin exhibited dose-dependent significant reversal of tactile allodynia in SNL rats as indicated in figure 2.
Aragen scientists have successfully established and validated the SNL model of the neuropathic pain using mechanical (Tactile) allodynia endpoint seen in humans. The rat model of SNL is robust, demonstrated reproducible pain behaviour for screening new analgesic drug candidates and studying pathophysiology or mechanisms of neuropathic pain.
To know more about Aragen’s pain models and In-vivo pharmacology services please write us at bd@aragen.com.
