

In vitro screening assays are critical aspects of the modern drug discovery process. Successful execution of these assays requires advanced instrumentation, plenty of consumables (tips, plates, reservoirs, troughs, etc.) and high-quality reagents (enzyme, DNA, peptide, buffer components, protein, etc.) in place. In cases where researchers have to screen large number of candidates, execution of these assays becomes time consuming and expensive. With the advent of several automation platforms, miniaturization of biochemical assays is one way through which the volume of reagents and quantity of the consumables are controlled thereby significantly reducing the time and running costs of the assays.
Here in this article, we report a study executed by the expert scientific personnel of the Aragen by miniaturization of biochemical assays to screen range of drug candidates against different target molecules.
Aragen Life Sciences initially supported the screening program using BiomekFxP liquid handler system as per the SOP shared by an European Biotech client. However, considering the excessive utilization of reagents and cumulative running costs of each assay, Aragen proposed to miniaturize the assay and proposed to automate various steps and to introduce ECHO550 liquid handling platforms. ECHO550 liquid handler is an innovative technology platform that uses acoustic droplet ejection for precise transfer of nanoliter-scale dispensing of samples in a much faster and more accurate way. It also enables tremendous reduction in the requirement of the compound for screening assays (~1mg or 20µl of 10mM DMSO stock for screening in 6-10 biochemical assays).
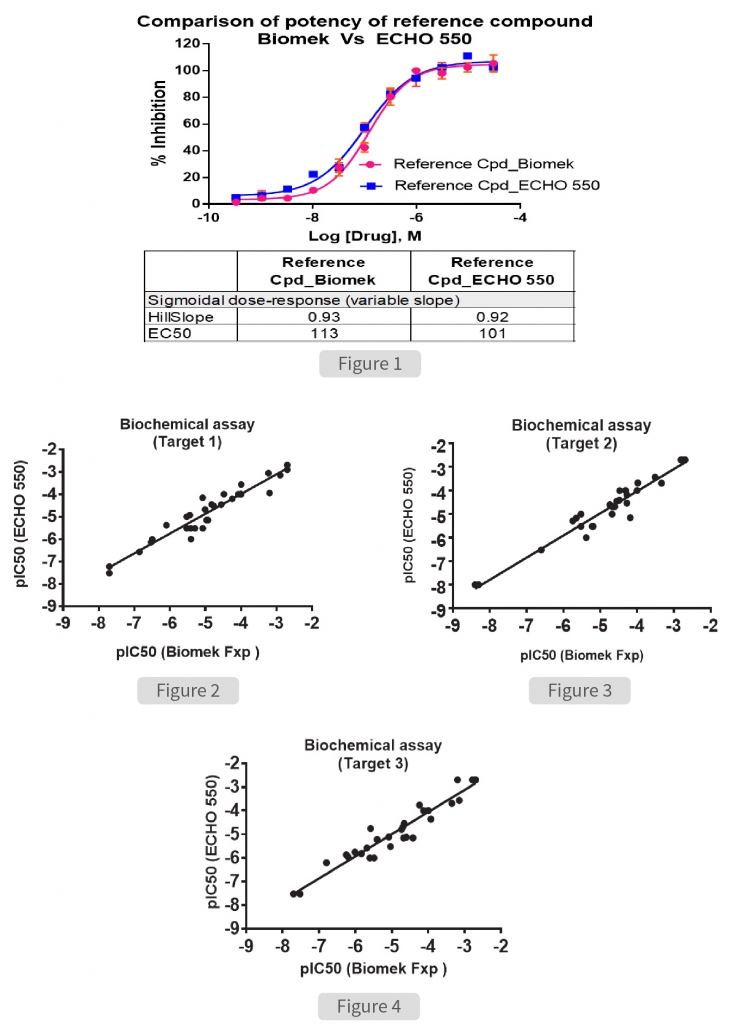
To assess the accuracy of miniaturization assay, we compared the data obtained with standard methodology performed using BiomekFxP. Initial investigation was carried out to determine the potency of assay specific standard reference compound to inhibit the activity of target enzyme (Figure 1). The half maximal inhibitory concentration (IC50) of the reference compound was determined to be 113nM and 101nM when the experiment was performed using the Biomek and ECHO 550 respectively. The overall assay quality, signal window and comparability of potency values obtained demonstrated that both methods were equally effective.
In the next set of experiments, biochemical assays were performed using Biomek Fxp and ECHO 550 to screen the range of drug molecules against three targets. To compare the efficiency of both the instruments the potency values of each drug molecules were compared. The results (Figure 2, Figure 3, Figure 4) demonstrate that the potency values obtained by both the methods fall in the range of regression slope demonstrating the comparable efficiency of both the methods.
Aragen achieved better results through direct dilution of compounds thereby increasing the sensitivity and robustness of assays and improved transfer quality through. We successfully miniaturized all the In vitro assays within a period of 3 weeks for this particular client and reduced the running cost of the assays without compromising on assay quality or sensitivity. With ~300 compounds screening/month in various assays, the miniaturization resulted in reduction of cost by up to ~75% as against the protocol suggested by client. The team was duly recognized and awarded by the client for their commitment and contribution.
Aragen Life Sciences is a leading R&D and manufacturing solutions provider for the life sciences industries worldwide. It offers end-to-end integrated or standalone solutions to advance small and large molecule programs from concept to commercialization. Established in 2001, the Company operates through a global network of six sites with a team of 3800+ employees and 450+ PhDs. Its expertise and experience have enabled over 450 customers (including 7 of the top 10 pharma companies globally) in advancing their research programs from early discovery through development and commercialization.
