

Glucagon-Like Peptide-1 (GLP-1) receptor agonists are transformative treatments for Type 2 diabetes and obesity. Comprehensive pharmacokinetic (PK) and anti-drug antibody (ADA) assessments are essential for optimizing dosing regimens, ensuring therapeutic efficacy, and maintaining safety. This whitepaper reviews the significance of PK and ADA evaluations in GLP-1 receptor agonist development and clinical use, highlighting key methodologies, challenges, and regulatory considerations.
GLP-1 receptor agonists are synthetic peptides that mimic endogenous GLP-1 to enhance insulin secretion, suppress glucagon, and reduce appetite. Key agents include albiglutide, dulaglutide, exenatide, liraglutide, and semaglutide. Their large peptide structure (Figure 1) makes them prone to enzymatic degradation, lowering bioavailability, and can trigger immunogenicity causing anti-drug antibodies (ADAs). These ADAs can neutralize effectiveness of GLP-1 agonists and cause adverse reactions. Understanding their pharmacokinetics and immune responses is essential for safe and effective treatment.
This paper examines the PK and ADA assessment processes of GLP-1 peptide receptor agonists, highlighting methodologies, regulatory guidelines, challenges, and clinical significance.
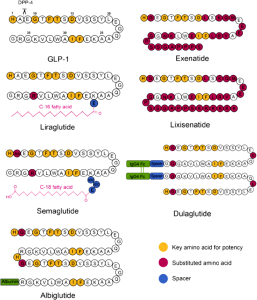
Figure 1: Structural comparison of GLP-1 receptor agonists. The amino acid sequences of GLP-1 and its analogues—Exenatide, Liraglutide, Lixisenatide, Semaglutide, Dulaglutide, and Albiglutide—are shown. Key structural features are highlighted to
indicate modifications relevant to potency and pharmacokinetics. (Source: Yu et.al; 2018).
Pharmacokinetic assessment is crucial to understand how GLP-1 receptor agonists are absorbed, distributed,
metabolized, and excreted, guiding optimal dosing, and ensuring therapeutic efficacy. The PK profile depends on
molecular structure, administration route, and pharmacological properties.
2.1. Administration Routes
The administration route significantly impacts the pharmacokinetics of GLP-1 receptor agonists by influencing the rate and extent of drug absorption, onset of action, bioavailability, and overall exposure.
2.2. PK Modeling
Pharmacokinetic (PK) modeling is a mathematical approach used to describe and predict the drug’s behavior in the body. Two primary modeling approaches are used for GLP-1 receptor agonists:
2.3. Bioanalytical Methods
Bioanalytical techniques are crucial for quantifying GLP-1 receptor agonists in biological samples. Key methods include:
2.4. Pharmacokinetic Parameters
The key PK parameters include:
2.5. Clinical PK Characteristics of GLP-1 Peptide Receptor Agonists
The PK characteristics of GLP-1 receptor agonists vary due to differences in their molecular structures, formulation, and metabolism. These variations directly impact absorption rates, half-lives, and bioavailability, ultimately influencing dosing regimens and the duration of therapeutic action:
2.6. In Vivo PK Studies and Their Relevance
Preclinical and clinical PK studies are essential for determining optimal dosing regimens. For example, pharmacokinetic modeling and dose escalation studies can help establish appropriate drug concentrations and administration schedules. PK modeling, including non-compartmental and compartmental approaches, helps in understanding drug exposure and clearance patterns. Animal models, such as rats and pigs, are often used due to their metabolic similarities to humans.
2.7. Challenges in PK Assessment of GLP-1 Analogues
3.1. Immunogenicity of GLP-1 Peptide Analogues
Immunogenicity refers to the ability of a drug to elicit an immune response, which may lead to the formation of anti-drug antibodies (ADAs). These antibodies can neutralize the drug, reduce efficacy, and cause allergic reactions. The immunogenic potential of GLP-1 peptide receptor agonists is influenced by their peptide structure and foreignness to the human immune system. The risk of ADA development varies among agents; for example, exenatide shows higher immunogenicity, while liraglutide and semaglutide have modifications that reduce this risk.
3.2. Mechanisms of ADA Formation
ADAs are typically formed when the immune system recognizes the receptor agonists as a foreign entity. The immune response may involve:
ADAs can increase drug clearance and reduce therapeutic benefit, sometimes causing hypersensitivity.
3.3. Methodologies for ADA Detection
ADA detection is an essential part of clinical trials and post-marketing surveillance:
3.4. Clinical Implications of ADAs
The presence of ADAs in patients receiving GLP-1 receptor agonists may lead to:
3.5. Regulatory Guidelines for ADA Assessment
The assessment of immunogenicity is a key part of the regulatory approval process for biologics. Regulatory bodies, including the FDA and EMA, require comprehensive immunogenicity testing during clinical development, including method validation, sample handling, and data traceability. Long-term post-marketing surveillance is mandated to monitor delayed or rare ADA-related adverse events and changes in pharmacokinetics.
Pharmacokinetic and anti-drug antibody (ADA) assessments are vital for optimizing dosing and safety of GLP-1 receptor agonists. Advances in PK modeling, immunogenicity testing, and personalized medicine will enhance treatment outcomes. Novel formulations and delivery methods are key to improving patient adherence and broadening therapeutic impact in diabetes and obesity. Continued innovation promises to boost the efficacy and accessibility of these important therapies.
