
The permeability of Proteolysis Targeting Chimeras (PROTACs) is a crucial determinant for their bioavailability and therapeutic efficacy. PROTACs are bifunctional molecules that induce targeted protein degradation by binding both a protein of interest and an E3 ligase. However, their larger size and complex structure often challenge their ability to efficiently cross biological membranes, making permeability a key consideration in drug discovery and development.
Passive permeability, which allows compounds to diffuse across membranes without the help of active transporters, is crucial for PROTACs. However, factors such as high molecular weight, polar surface area, and exposed hydrogen bond donors frequently hinder this process.
Unlike traditional small molecules that follow Lipinski’s Rule of 5 for predicting permeability and oral bioavailability, PROTACs often exceed these guidelines, making their transport across biological barriers challenging. Therefore, choosing the right cell models to evaluate their permeability is essential.
In this study, we evaluated the permeability characteristics of three PROTACs—ARV-771 (ALS-010), ARV-110 (ALS-011), and KT-474 (ALS-012) along with three quality control (QC) compounds: Propranolol (highly permeable), Atenolol (low permeable), and Digoxin [a P-glycoprotein (P-gp) substrate] – using three different cell lines:
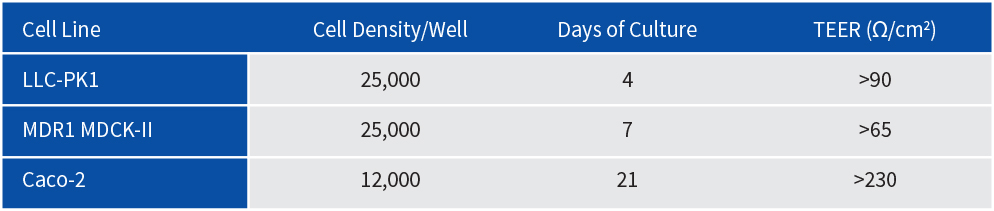
Permeability assays were performed using Millicell 96-transwell plate, with cells cultured under standard conditions.
The following parameters were measured:
Cell Culture Parameters
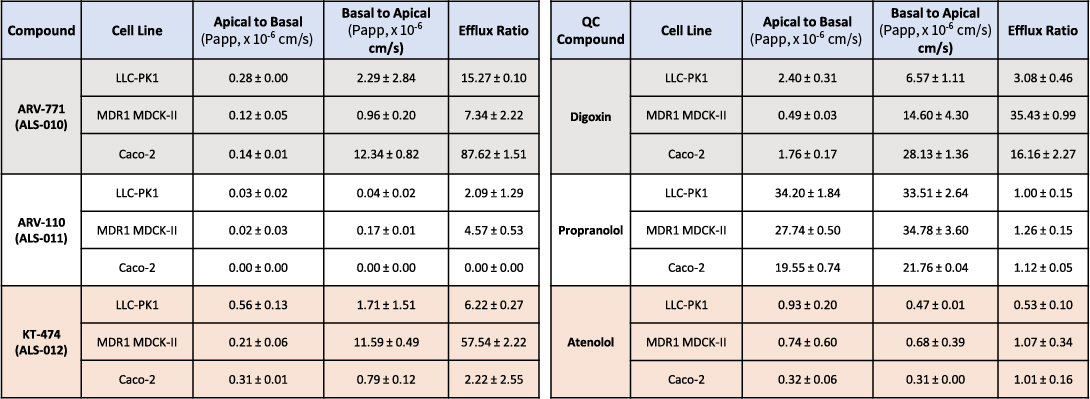
The study compared the permeability of PROTACs across three cell lines—LLC-PK1, MDR1 MDCK-II, and Caco-2—with the hypothesis that LLC-PK1 offers advantages over the other two models. The findings highlight differences in permeability, efflux ratios (Table 1), and recovery rates (Table 2), providing insights into the suitability of each cell line for assessing PROTAC transport and absorption.
Permeability Profiles and Efflux Ratios
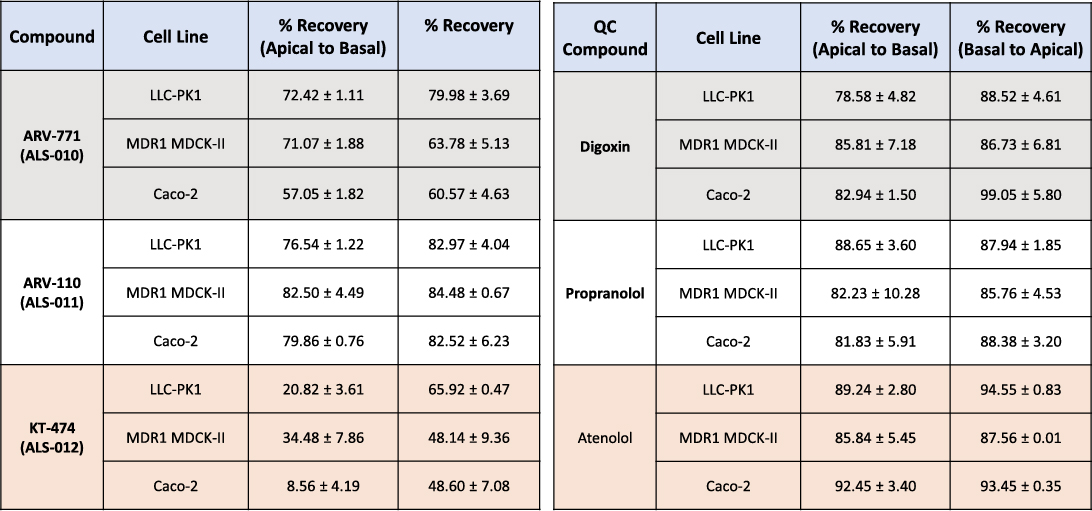
Recovery Rates
Advantages of LLC-PK1 Cell Line
Utility of MDR1 MDCK-II Cell Line
Challenges with Caco-2 Cell Line
PROTAC-Specific Insights
The case study highlights that:
Aragen Life Sciences provides comprehensive, reliable, and efficient DMPK services tailored to meet drug discovery needs. We offer:
