

The demand for rapid, reliable, and scalable production of biologics has driven innovation in cell line development (CLD). Transposase technologies continue as a transformative solution, enabling controlled, high-efficiency gene integration that delivers stable, high-yielding mammalian cell lines. For Contract Research, Development, and Manufacturing Organizations (CRDMOs), adopting transposase platforms means not only accelerating timelines and reducing risk, but also ensuring reproducible product quality and robust scalability from early development through commercial manufacturing.
This whitepaper outlines the technical foundations of transposase technologies, highlighting their impact on accelerating development, improving consistency, and enabling scalable biologic production. It also reviews how CRDMOs apply these tools to optimize processes and outcomes for clients.
In the biopharmaceutical industry, the creation of stable mammalian cell lines (especially CHO cells) is a critical step for the production of monoclonal antibodies, recombinant proteins, and other biologics. Achieving consistent gene expression is essential for product quality, regulatory compliance, and manufacturing efficiency.
Traditional gene integration methods, such as random plasmid integration or viral transduction, insert the gene of interest at unpredictable locations in the genome. This randomness can lead to:
Transposase technologies address these challenges by leveraging the natural ability of transposons (“jumping genes”) to insert DNA into the genome in a controlled and efficient manner.
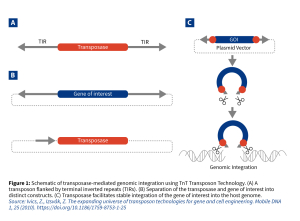
Transposase systems use engineered “jumping genes” or transposons—DNA elements flanked by inverted terminal repeats (ITRs)—which are mobilized into the host genome by a transposase enzyme provided during transfection, typically as mRNA. This targeted “cut-and-paste” mechanism facilitates semi-targeted integration into transcriptionally active regions, minimizing random insertion drawbacks such as fragmentation, concatemerization, and rearrangements. As a result, the gene of interest (GOI) is stably inserted as intact expression cassettes, promoting homogeneous, stable, and predictable expression across cell populations (Figure 1).
| Feature | Random Integration | Viral Vectors | Transposase Technologies |
| Integration Site | Unpredictable | Semi-random | Preferentially active sites |
| Copy Number | Variable, often multiple | Variable | Often single, intact |
| Expression Consistency | Low | Moderate | High |
| Genetic Stability | Moderate | Moderate | High |
| Clone Screening Requirement | Extensive | Moderate | Minimal |
| Regulatory Complexity | Moderate | High (viral elements) | Low |
1. Efficient and Precise Gene Integration
Transposases introduce a level of precision that traditional random integration methods lack. The process begins by introducing a transposon vector containing the gene of interest, and the transposase enzyme facilitates the insertion of this gene into a target genomic site.
This precision enhances the likelihood of creating stable, high-expressing clones in a fraction of the time it would take with traditional methods.
2. Accelerated Clonal Selection and Expansion
Once transgenes are integrated, selecting, and expanding high-expressing clones is often the most time-consuming step in cell line development. Transposase systems reduce this burden by generating highly productive cell populations early in the process.
This rapid, reliable clonal expansion reduces the overall time to development and accelerates the transition from early-stage development to manufacturing.
3. High-Yield and Consistent Expression
Transposase technologies enable the creation of high-yield cell lines that exhibit consistent and reproducible protein expression over extended production cycles.
The result is superior product consistency—a key consideration for regulatory compliance and commercial success in biologic production.
1. Scalability and Transition to Manufacturing
One of the most critical challenges in biomanufacturing is scaling up cell lines from preclinical stages to commercial production. Transposase systems, due to their high efficiency and consistency, facilitate seamless scale-up.
CRDMOs can confidently move from small-scale to large-scale production, ensuring that regulatory expectations for product quality and reproducibility are met without the need for complex adjustments during scale-up.
2. Optimized Resource Utilization and Cost Reduction
By accelerating the cell line development process and increasing clone stability, transposase technologies reduce the resources required for selection, screening, and validation. This contributes directly to cost reductions in the early stages of biologic development.
For CRDMOs, these efficiencies create opportunities to offer more competitive pricing, while still ensuring high-quality outcomes for clients.
Aragen Bioscience demonstrates the power of transposase technologies through its CHOMax™ platform with FutꚙKO—a next-generation cell line development (CLD) system engineered for speed, reliability, and high productivity. By integrating proprietary TnT Transposon Technology for precise and efficient gene insertion with glycoengineered CHO host lines, Aragen enables faster development timelines, stable high-yield expression, and consistent product quality.
The platform is designed to fast-track IND readiness by combining productivity, precision, and confidence in every stage of development. Its FutꚙKO glycoengineered GS knockout cell line supports full afucosylation, enhancing antibody-dependent cellular cytotoxicity (ADCC) and improving therapeutic potency—making it ideal for monoclonal antibodies and next-generation biologics requiring superior effector function and performance. The CHOMax™ platform with Fut∞KO enhances ADCC activity, expanding the therapeutic window, reducing dosage requirements, and minimizing undesirable side effects.

This efficient, validated workflow (Figure 2) supports rapid, high-yield cell line generation with strong clonal stability and reproducible performance.
Transposase technologies represent a significant advancement in cell line development, offering faster, more stable, and higher-yield solutions essential for modern biomanufacturing. By leveraging these technologies, CRDMOs empower their clients to reduce timelines, lower costs, and ensure consistent product quality—critical factors in accelerating biologics development and commercialization.
Partnering with CRDMOs that harness transposase technologies provide a strategic advantage in meeting the growing demands of the biopharmaceutical industry, enabling efficient, scalable, and reliable biologic production.
With consistent regulatory acceptance and high titers from grams to kilograms, Aragen’s CHOMax™ platform supports a wide range of applications—from therapeutic biologics to diagnostics and bioanalytical reagents. By integrating TnT transposase technology and FutꚙKO glycoengineering, Aragen provides a faster, more predictable path from discovery to IND and beyond, ensuring productivity, precision, and confidence at every stage of cell line development and manufacturing. We offer:
Whether you’re developing monoclonal antibodies, complex fusion proteins, or gene therapy vectors, our integrated platform is designed to reduce timelines, improve consistency, and scale with your program from early development through commercial manufacturing.
Partner with us to streamline your cell line development and unlock the full potential of your biomanufacturing pipeline.
