

Evolving global regulations have made nitrosamine control essential across the pharmaceutical industry. Regulatory bodies such as the FDA, EMA, WHO, and ICH (M7, Q2(R2), Q14) now require highly sensitive, scientifically defensible, and fully validated methods capable of quantifying trace-level nitrosamines in complex drug matrices. Meeting these standards demands advanced technology, specialized expertise, and a systematic approach to method development.
Aragen’s, Analytical Solutions Laboratory (ASL) delivers on these requirements with LC–MS/MS method development and validation workflows aligned to current regulatory expectations, supporting innovators, generics, and biotech organizations with reliable nitrosamine assessment and control solutions.
Regulatory limits for nitrosamines—including NDMA, NDEA, NMBA, and NDSRIs—continue to tighten, with sub-ng/mL and ppb-level detection often required. Achieving such sensitivity calls for optimized sample preparation, high-efficiency extraction, and precisely calibrated instrumentation.
To meet these standards, ASL develop methods that incorporate both solid phase extraction (SPE) and liquid–liquid extraction (LLE), evaluating solvents, pH adjustments, and partitioning behaviour according to the physicochemical properties of each analyte and matrix. These optimized workflows ensure recovery efficiencies exceeding 90% and matrix effects below 10%, enabling consistent quantification even in challenging drug substance or drug product matrices.
Regulatory expectation for data reliability and reproducibility requires chromatographic systems that deliver stable retention times, clean peak shapes, and high ionization efficiency. To meet these requirements, our methods employ reversed-phase C18 and polar-embedded columns with carefully tuned gradient programs. Electrospray ionization in positive mode, combined with finely tuned multiple reaction monitoring (MRM) transitions and collision energies, ensures clear, interference-free detection.
Beyond standard linearity criteria, our methods consistently demonstrate high signal-to-noise ratios, minimal background interference, and stable responses across the full analytical range—ensuring precise, defensible quantitation from LOQ through release-specification levels.
Authorities mandate the use of scientifically defendable quantitation strategies that account for matrix effects, instrument variability, and extraction bias. To meet this requirement, we employ isotope-labelled internal standards (ILIS) for every nitrosamine analyte. Matrix-matched and solvent-based calibration approaches are applied to demonstrate accuracy and recovery, and all data handling adheres strictly to ALCOA+ principles (Figure 1).
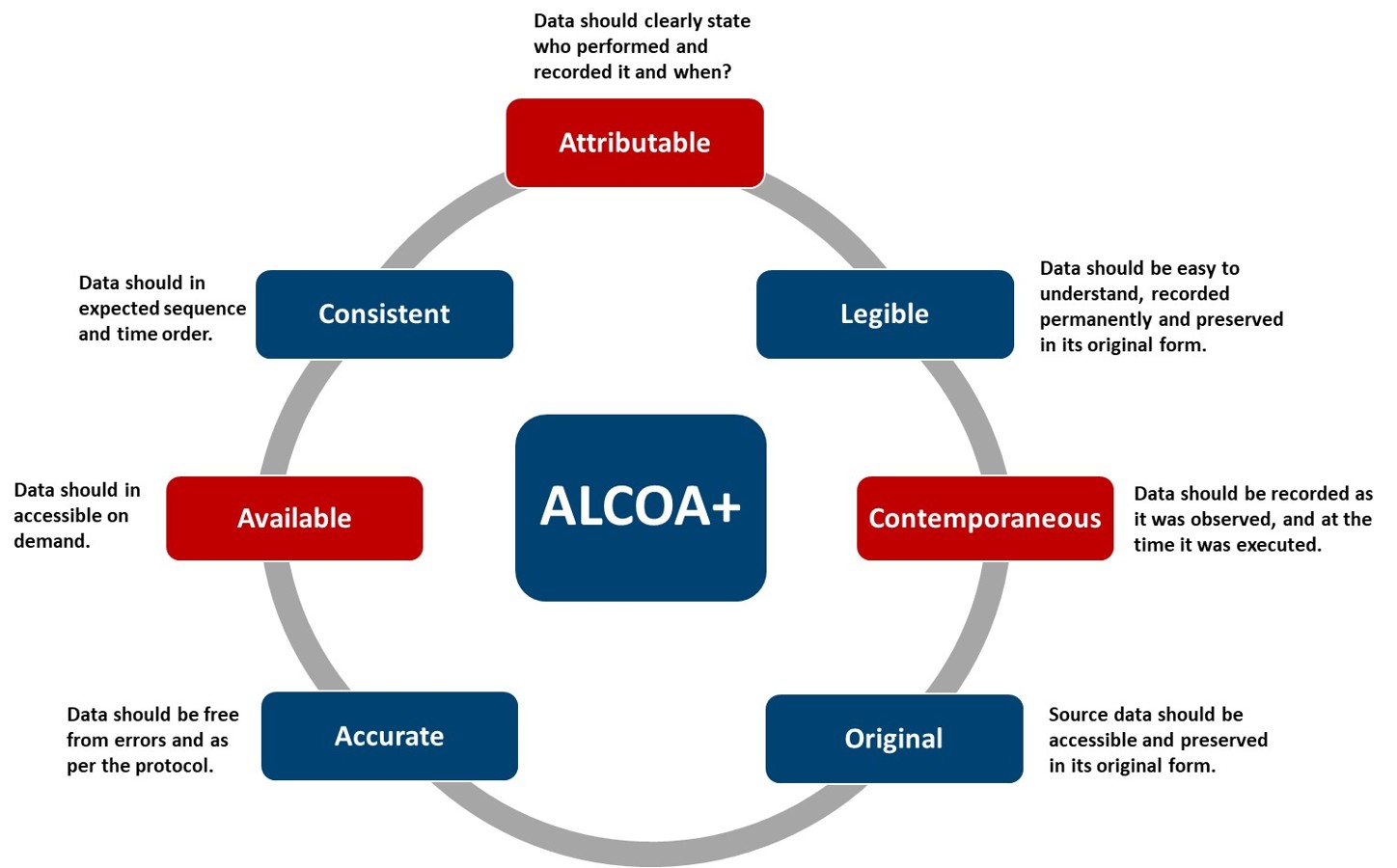
Figure 1: ALCOA+ Principles for Data Integrity—Attributable, Legible, Contemporaneous, Original, Accurate, plus Consistent, Available, Complete. These elements ensure compliance, reliability, and trustworthiness of data in regulated environments.
Validation packages must withstand regulatory scrutiny and ensure compliance at every step while demonstrating method suitability across specificity, precision, accuracy, robustness, stability, and more. Our validation framework, executed under GLP and ISO/IEC 17025 accreditation, is designed to meet these exact criteria. Statistical evaluations and comprehensive documentation ensure each method is defensible, auditable, and ready for global submissions.
As regulatory authorities move toward broader surveillance of nitrosamine families, laboratories must handle multiple analytes efficiently. To address this requirement, we have established multi-nitrosamine LC–MS/MS methods capable of quantifying up to eight nitrosamines within a single run—significantly reducing turnaround times while maintaining accuracy and sensitivity (Figure 2).
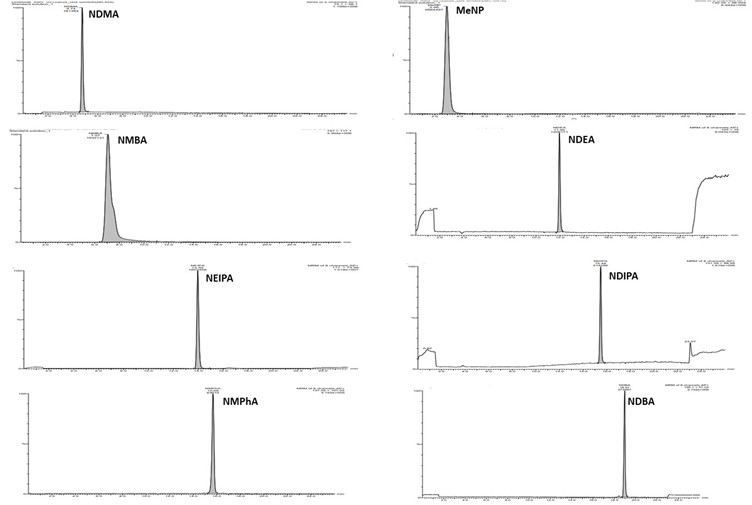
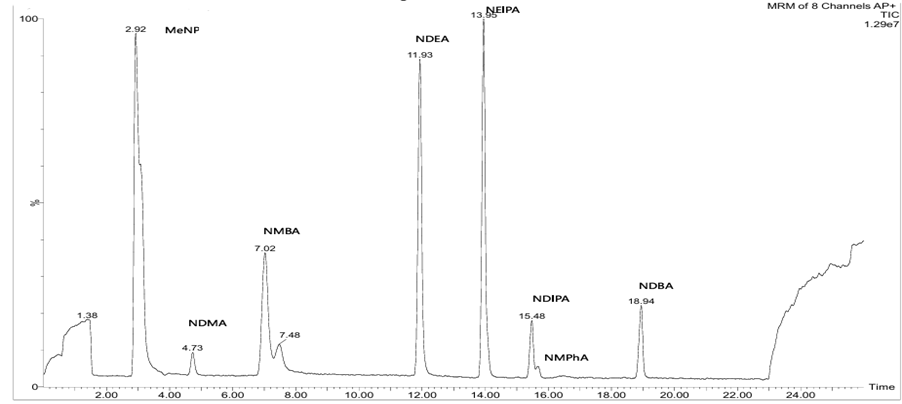
Figure 2: LC–MS/MS method for multi-nitrosamine analysis. The top panel displays individual mass spectra for each nitrosamine (MeNP, NDMA, NMBA, NDEA, NEIPA, NDIPA, NMPhA and NDBA), while the bottom panel shows an overlay of the eight nitrosamines detected within a single chromatographic run.
Current regulations extend beyond testing—they require a comprehensive understanding of nitrosamine formation and prevention. ASL provides full risk assessments that include:
These insights help manufacturers build effective control strategies while protecting product quality and supply continuity.
All analytical activities undergo independent Quality Assurance review to ensure traceability, data integrity, and regulatory compliance. Our collaborative approach keeps clients informed throughout the entire project lifecycle—from feasibility studies through method transfer—supported by interim progress reports and technical discussions. Continuous feedback fosters delivery of scientifically sound, regulatory-ready, and client-aligned analytical solutions.
Precise and reliable analytical methods are essential to ensure the safety, quality, and regulatory compliance of pharmaceutical products, particularly when detecting trace-level impurities such as nitrosamines. Robust LC–MS/MS techniques offer sensitive, specific, and reproducible quantification necessary for comprehensive risk assessment and quality control.
At Aragen, with a dedicated team of experienced scientists and advanced instrumentation such as LC-MS/MS and GC-MS/MS, Analytical Solutions Laboratory (ASL) delivers GMP-compliant nitrosamine analyses at ppm, ppb, and ppt levels—including cleaning-validation support—providing clients with rigorous, regulatory-ready results.
Partner with us for precision and compliance—take your molecule from analysis to approval with Aragen.
