
Peptides are rapidly emerging as critical therapeutic agents and biomarkers, requiring highly sensitive and reliable analytical/ bioanalytical methods for their quantification in complex biological matrices. This whitepaper discusses the key challenges in peptide bioanalysis—such as low dose detection, signal interference, recovery inefficiencies, and chromatographic complexities—and presents robust LC-MS/MS workflow optimizations developed by Aragen’s DMPK team. By refining sample preparation, chromatographic conditions, and detection parameters, we have established sensitive, reproducible assays that enhance data quality and accelerate early-stage drug discovery. Our validated approaches empower informed decision-making and facilitate the selection of promising peptide drug candidates, supporting innovative peptide therapeutics development.
Peptides, composed of short amino acid sequences, represent a versatile and growing class of therapeutic molecules with applications across numerous disease areas. Their effective engagement with biological targets underscores their importance in both drug development and biomarker discovery. However, accurate quantification of peptides in biological samples such as plasma or tissue remains analytically challenging due to their typically low concentrations and the complex nature of biological matrices.
Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) has become the gold standard for peptide bioanalysis, offering unparalleled sensitivity and specificity. Despite this, peptide quantification is complicated by issues including signal interference from similar molecules, low recovery during sample processing, and demanding chromatographic requirements.
At Aragen, our DMPK scientists are dedicated to overcoming these challenges by developing and validating optimized LC-MS/MS workflows tailored to peptide bioanalysis. This whitepaper highlights the technical hurdles encountered and the innovative solutions we have implemented to ensure accurate, reproducible peptide quantification—ultimately supporting the acceleration of peptide drug discovery and development pipelines.
1. Low Dose and Signal Interference
These challenges were addressed through method optimization and careful selection of analytical parameters, ensuring reliable quantification even at low concentrations.
2. Blank Interference
Blank interference arises from non-specific signals or background noise originating from the sample matrix, reagents, or instrument carryover. This interference can obscure true analyte detection, especially when the target concentration is near the lower limit of quantification (LLOQ).
To address this, we evaluated several strategies to reduce background interference without compromising analytical sensitivity. Effective approaches included:
Together, these steps improved signal-to-noise ratios and minimized false positives, ensuring more reliable quantification. Thorough sample pre-treatment and cleanup are critical to eliminate potential interferences and maintain assay robustness. Representative chromatograms illustrating blank interference and internal standard response are shown in Figure 1.
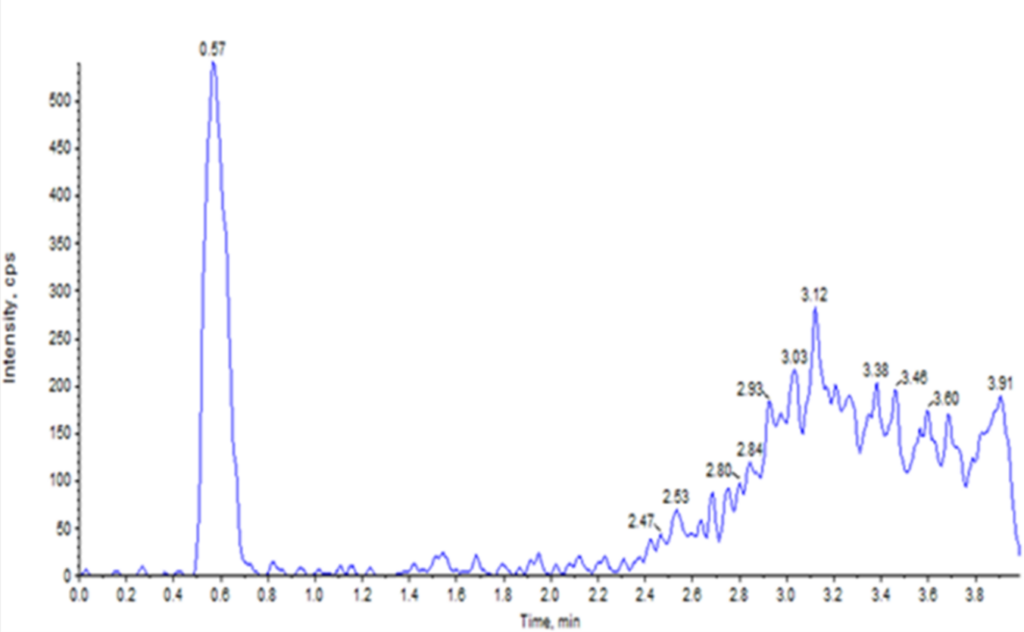
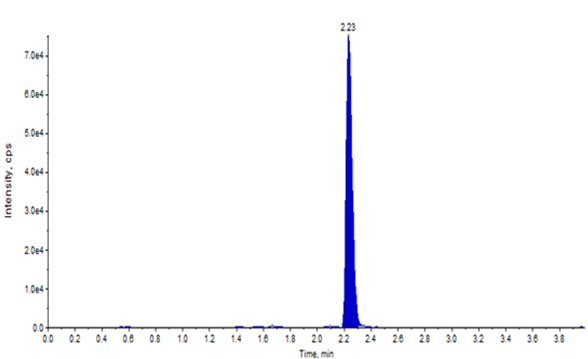
Figure 1: Representative chromatograms for blank (top) and internal standard (bottom).
3. Recovery Efficiency
Peptide recovery from biological samples is a critical factor in drug development and pharmacokinetic analysis. However, achieving high recovery rates is often hindered by physicochemical challenges, leading to significant losses of peptide quantity and quality. These losses impact downstream applications such as mass spectrometry, bioassays, and therapeutic formulations.
We identified common challenges, their underlying causes, and their impacts, and implemented targeted approaches to improve recovery efficiency (Table 1).
| Challenge | Reason | Impact | Solution |
| Adsorption Losses | Peptides bind to surfaces of containers and tips | Significant peptide loss | Use low-binding pipette tips and polypropylene plates |
| Degradation | Enzymatic or chemical breakdown of peptides | Loss of peptide integrity | Acidify samples (e.g., with formic acid) to stabilize |
| Solubility Issues | Poor solubility in solvents | Precipitation, incomplete recovery | Optimize buffer composition, adjust pH, apply gentle heating |
| Peptide Aggregation | Aggregation under certain conditions | Reduced recovery and analysis difficulty | Modify ionic strength and pH to prevent aggregation |
4. Chromatographic Conditions
Peptide analysis by liquid chromatography (LC), especially in high-throughput settings, presents technical challenges that can affect data quality and reproducibility. These challenges arise from the intrinsic properties of peptides and their interactions with chromatographic systems.
Key issues encountered during peptide separation and injection along with the strategies applied to mitigate them are mentioned below (Table 2):
| Challenge | Reason | Solution |
| Poor Peak Shape | Strong peptide-column interactions; similar peptides (physicochemical properties) | Use ACQUITY UPLC® HSS T3, 1.8 μm, 3×30 mm column for sharp peak shapes; optimize mobile phases. |
| Carryover | Strong binding to column or injector surfaces | Implement longer gradient runs at low flow rates to reduce carryover |
| Sample Loss During Injection | Adsorption to vial or injector surfaces | Use low-binding sample plates; minimize sample handling to preserve peptide integrity |
These strategies significantly enhanced chromatographic performance, improving reproducibility and sensitivity in peptide analysis workflows, particularly when handling complex peptide mixtures.
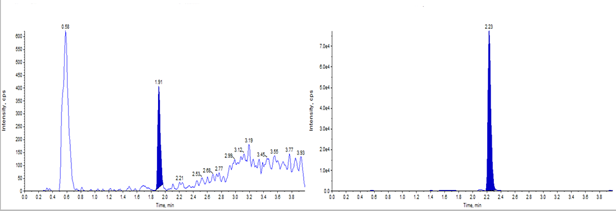
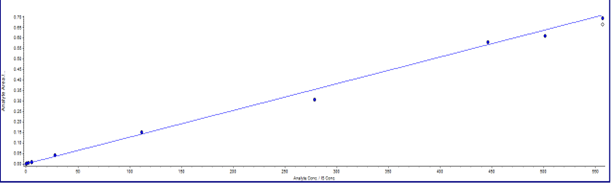
Figure 2: Representative chromatograms for test sample (left) and internal standard (right) at the Lower Limit of Quantification (0.1 ng/mL) [A], along with linearity plot demonstrating method performance across the calibration range [B].
By incorporating these optimized strategies, a high-resolution LC-MS/MS workflow was developed that achieved rapid and sensitive peptide profiling, demonstrating excellent recovery, consistent retention times, and LLOQs at or below 0.1 ng/mL (Figure 2). Linearity across the calibration range was confirmed through method validation, as shown in the linearity plot (Figure 2). Most of the peptides analyzed in this study were synthetic, with molecular weights ranging from 1,400 to 3,000 Daltons.
This workflow enabled identification and mitigation of metabolic liabilities, improved plasma stability, and facilitated elucidation of soft spots across multiple peptide candidates. Integration of these methods into drug discovery process has accelerated decision-making and supported the progression of lead candidates with favorable pharmacokinetic profiles (Figure 3).
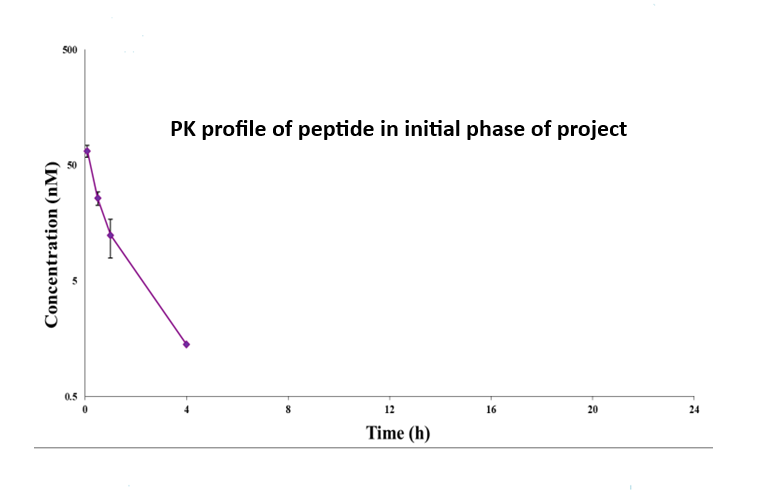
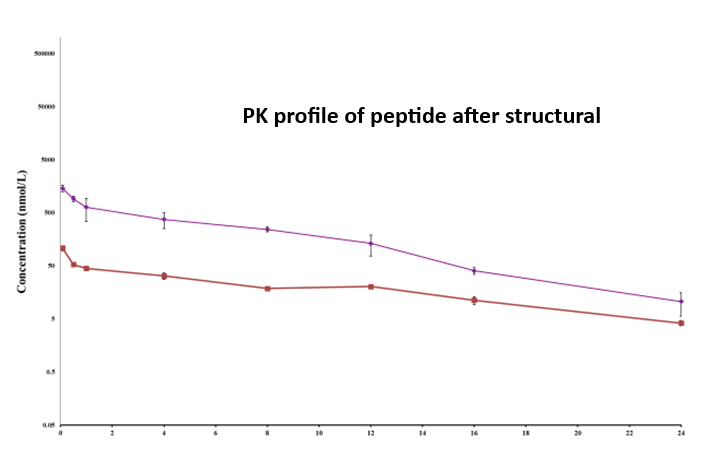
Figure 3: Pharmacokinetic (PK) profiles of the peptide across two project phases. The top panel illustrates the PK behavior during the initial phase, while the bottom panel shows the profile following structural modification. Concentration (nM) is plotted against time (h), demonstrating the impact of molecular changes on systemic exposure and clearance.
LC-MS/MS is an indispensable tool in peptide bioanalysis, offering the sensitivity and specificity required for low-dose quantification and complex biological matrices (plasma, serum, or tissue homogenates). Achieving a sensitive and specific LC-MS/MS method that generates reliable and reproducible data requires addressing several analytical challenges, including:
Advances in method development, including high-efficiency columns, tailored mobile phases, and automated sample handling, at Aragen significantly improved throughput and reliability in peptide bioanalysis. Standardizing analytical workflows also facilitates cross-study comparisons and supports regulatory compliance.
Collectively, these advancements enhance drug discovery efficiency and enable biomarker validation, ultimately accelerating the development of peptide-based therapeutics.
Aragen is well-equipped to support bioanalysis of peptides and emerging therapeutic modalities through advanced infrastructure and specialized expertise.
