

High-Content Screening (HCS) is a transformative technology in modern drug discovery, enabling automated, high-throughput, multiparametric analysis of cellular responses at single-cell resolution. By converting complex biological images into quantitative data, HCS supports informed decision-making across discovery and translational research. Aragen deploys advanced HCS platforms to streamline hit identification, lead optimization, toxicity profiling, and mechanism-of-action studies across diverse two-dimensional (2D) and three-dimensional (3D) models. This whitepaper highlights how HCS accelerates phenotypic screening, hit validation, mechanism-of-action studies, and lead optimization, offering actionable insights for smarter drug discovery decisions.
Therapeutic targets and disease biology are becoming increasingly complex, and traditional single-endpoint assays often fall short. High-Content Screening (HCS) addresses these challenges by combining automated microscopy, advanced optics, and quantitative image analysis to generate rich, multiparametric datasets at scale.
By preserving spatial and temporal context, HCS enables nuanced characterization of cellular phenotypes in biologically relevant systems. Contemporary platforms support high-resolution analysis of fixed and live cells in both two-dimensional (2D) and three-dimensional (3D) models. Features such as confocal imaging, environmental control, and kinetic acquisition allow detailed interrogation of subcellular organization, protein localization, and dynamic cellular events.
Integrating with OMICS datasets and computational frameworks, HCS delivers deep phenotypic insight, supporting systems-level drug discovery. This integration enables comprehensive phenotypic screening, mechanism-of-action studies, and translational decision-making, leading to more informed decisions about therapeutic development strategies.
Identifying Hits and Pathways Efficiently
HCS facilitates multiplexed fluorophore analysis which enables elucidating complex intracellular molecular networks. In general, cells or proteins are stained with fluorescent probes specific to the assay—such as propidium iodide/ DAPI, caspase-based dyes to stain the apoptotic cells or target protein specific staining dyes or antibodies—followed by HCS imaging and advanced software analysis.
The technology’s high imaging speed, compatibility with 96- and 384-well formats, and robust analytical capabilities position HCS as optimal for primary hit screening. Multiple fields per well and replicates help ensure statistical significance, typically employing lower-magnification objectives to optimize throughput and manage data efficiently. HCS experiments generate substantial data volumes, and associated analysis software enables clear, actionable insights. This technology can support both single time-point endpoint studies and kinetic imaging modes to track phenotypic effects over time.
Hit Validation and Mechanistic Insights
Beyond primary screening, HCS can be used for rigorous hit validation through orthogonal assay strategies, including mutant models, stable or engineered cell lines, and pathway perturbation in these models. These approaches confirm biological relevance while reducing false-positive attrition.
Mechanistic investigation is a particular strength of HCS. Phenotypic profiling approaches, such as cell painting, enable unbiased assessment of compound-induced cellular states. HCS phenotypic profiling can capture thousands of quantitative features per cell—such as morphology, organelle distribution, and intensity patterns—generating signatures that reveal functional relationships, differentiate compound classes, and illuminate mechanisms of action with high confidence.
Tracking Dynamics: Live-Cell vs. Fixed-Cell Assays
HCS assays include both fixed-cell and live-cell formats across 2D and 3D systems. Fixed-cell assays allow broader use of dyes and antibodies, while live-cell assays rely on live cell-compatible probes or genetically encoded reporters.
Each format provides complementary advantages. Live-cell imaging supports real-time tracking of dynamic processes such as receptor internalization, migration, invasion, and cell fate. Fixed-cell assays enable co-localization and pathway analysis at defined time points. Optimized imaging balances temporal resolution, phototoxicity, and data volume.
Both approaches have advantages and limitations. For example, receptor internalization studies can be performed using live cells expressing a fluorescently tagged receptor, provided that the fusion protein does not alter cellular metabolism, localization, or protein function. Following stimulation, receptor trafficking through endosomal or lysosomal pathways can be monitored in real time and validated using co-localization with organelle-specific markers.
Alternatively, the same study can be conducted using fixed cells without prior labeling. Cells are exposed to stimuli for defined time points, fixed, and stained with antibodies against the receptor and organelle markers. Live-cell imaging is essential for assays such as migration, invasion, and cell tracking. Live-cell dyes (e.g., calcein) combined with rapid image acquisition enable real-time analysis of dynamic processes. Given the large data volumes generated, imaging parameters—including field number, replicates, dimensionality, channels, and time points—must be carefully optimized.
3D Specimens Imaging
At Aragen, we leverage High-Content Screening (HCS) platforms to enable high-throughput imaging and analysis of complex 3D biological models. These advanced systems closely mimic, in vivo physiology, and are increasingly critical for translational research.
To ensure accurate and detailed visualization in these models, confocal imaging plays a key role by enabling optical sectioning of thick specimens, which improves resolution and structural clarity. Imaging depth and signal quality can be further optimized using high magnification water immersion objectives and optical clearing techniques to minimize light scattering. Continuous advancements in HCS technology have significantly enhanced the feasibility and efficiency of high-throughput screening in 3D models.
Cell painting represents a robust HCS-based phenotypic profiling approach. This assay employs a defined set of fluorescent probes to label major cellular compartments, including the nucleus, cytoskeleton, mitochondria, Golgi apparatus, endoplasmic reticulum, and plasma membrane.
Images acquired through HCS are analyzed to extract thousands of quantitative features per cell. These features are used to generate phenotypic signatures that reflect compound-induced cellular states. Compounds with shared mechanisms of action often produce similar phenotypic profiles, enabling classification, drug repurposing, and unbiased mechanism-of-action studies.
Advanced image analysis is central to the success of HCS-based experiments. Each cell contains rich quantitative information that can be extracted using sophisticated analysis pipelines. Standard workflows include image correction, segmentation, quality filtering, and object-based quantification.
Beyond basic cellular metrics, advanced analysis enables assessment of organelle organization, protein distribution, co-localization, cell motility, neurite architecture, and spatial fluorescence patterns. These readouts can be performed at both population and single-cell levels with high efficiency and reproducibility.
One example of this capability is cell painting, which demonstrates the power of computational image analysis. Using AI-enabled tools, high-dimensional feature sets are extracted and subjected to statistical modeling and clustering. Integration of phenotypic data with OMICS datasets further enhances biological interpretation and supports disease modeling and mechanism-of-action studies.
Advanced image analysis workflows (Figure 1) deliver high-quality, reproducible insights from HCS experiments.
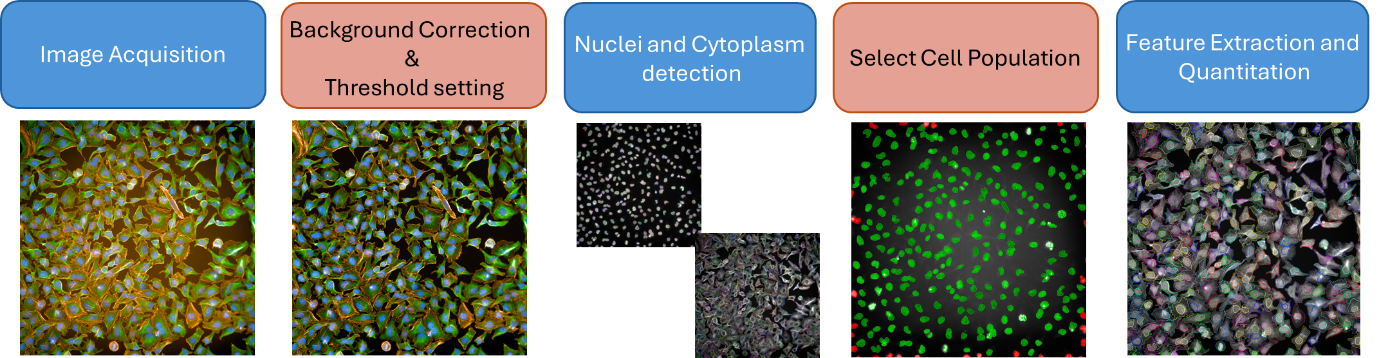
Figure 1: Overview of a typical HCS image analysis workflow.
While plate readers and flow cytometry remain valuable tools, HCS provides distinct advantages through multiparametric, spatially and temporally resolved measurements. This multiparametric capability increases the predictive power and biological relevance in drug discovery assays. For instance, induced pluripotent stem cell (iPSC) differentiation can be assessed by flow cytometry or immunoprecipitation; however, HCS enables concurrent evaluation of marker expression alongside morphological changes.
Furthermore, HCS enables integrated assessment of protein expression, subcellular localization, cellular morphology, and dynamic processes, neurite outgrowth, intracellular trafficking, viral entry, cell–cell interactions, and biomolecular condensate formation with high fidelity (Figure 2).
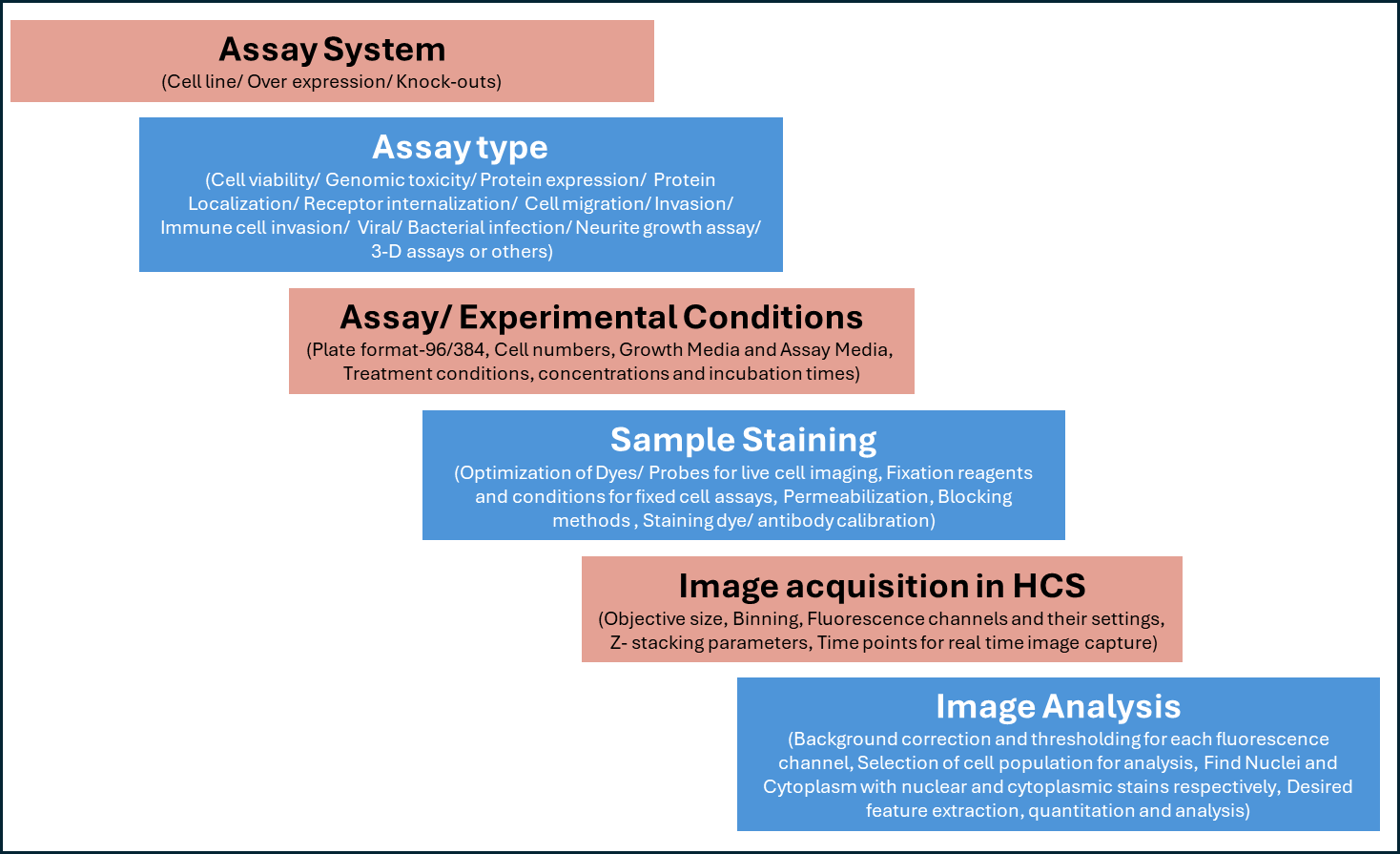
Figure 2: Chart showing assay development steps for HCS based experiments.
A key challenge in HCS experiments is data management, since imaging parameters such as magnification, number of channels, z-stacks, and time points can rapidly increase data volume. Careful experimental design is essential to maximize information content while maintaining manageable data sizes.
High-throughput HCS workflows also require optimized automation for cell seeding, reagent handling, and staining. Washing steps must be carefully controlled to minimize cell loss, particularly for suspension or weakly adherent cell types. In such cases, extracellular matrix–coated formats can improve assay robustness, provided that cellular morphology and function remain unaltered. It is critical to address these technical and operational challenges by implementing best practices in experimental design, automation, and assay optimization.
High-Content Screening has emerged as a highly adaptable and powerful platform for modern drug discovery. Its ability to combine high-throughput imaging with multiparametric, single-cell analysis enables robust hit identification, lead optimization, mechanism-of-action studies, and toxicity assessment.
By integrating advanced imaging modalities, physiologically relevant model systems, and sophisticated data analysis, HCS continues to expand the scope and depth of image-based drug discovery. These capabilities underscore its critical role in advancing translational research and accelerating the development of next-generation therapeutics.
With decades of experience and world-class infrastructure, Aragen provides comprehensive assay development and high-content screening services that accelerate drug discovery. Our expertise in advanced imaging, data analysis, and integrated workflows ensures faster and more informed decision making for our clients in their drug discovery programs.
