
Small molecule inhibitors play a crucial role in drug discovery, particularly for targeting disease-associated proteins in oncology. Early-stage research efforts focus on identifying compounds with both potent biological activity and favorable pharmacokinetic properties. However, translating strong biochemical potency into meaningful cellular activity and desirable in-vivo efficacy often proves challenging, as issues like poor cellular permeability, rapid metabolic clearance and poor oral bioavailability can undermine compound effectiveness. This study was designed to systematically address these limitations by optimizing lead molecules for improved cellular activity, metabolic stability and pharmacokinetics, ultimately achieving significant tumor growth inhibition in preclinical mouse models and facilitating progress towards the development of targeted cancer therapeutics.
The program’s main challenge was to identify up to three small molecule inhibitors that could achieve more than 75% tumor growth inhibition (TGI) in mouse models within an accelerated timeline (8–10 months). Initial lead compounds, despite showing strong biochemical potency, failed to demonstrate sufficient cellular activity, and had poor pharmacokinetic properties—including high in-vivo clearance and low oral bioavailability. Achieving robust cellular potency (IC50 < 250 nM) and improved PK profiles was critical to advancing these compounds toward preclinical development.
To address these issues, the Aragen Integrated Drug Discovery (IDD) team implemented a systematic structure-activity/property relationship (SAR/SPR) investigation. Structural modifications were introduced at several critical sites of the molecule (Figure 1), including:
Modifications made to the linker and acid group did not improve cellular activity. However, targeted changes to the HetCy ring and the LHS Q-group significantly enhanced cellular potency.
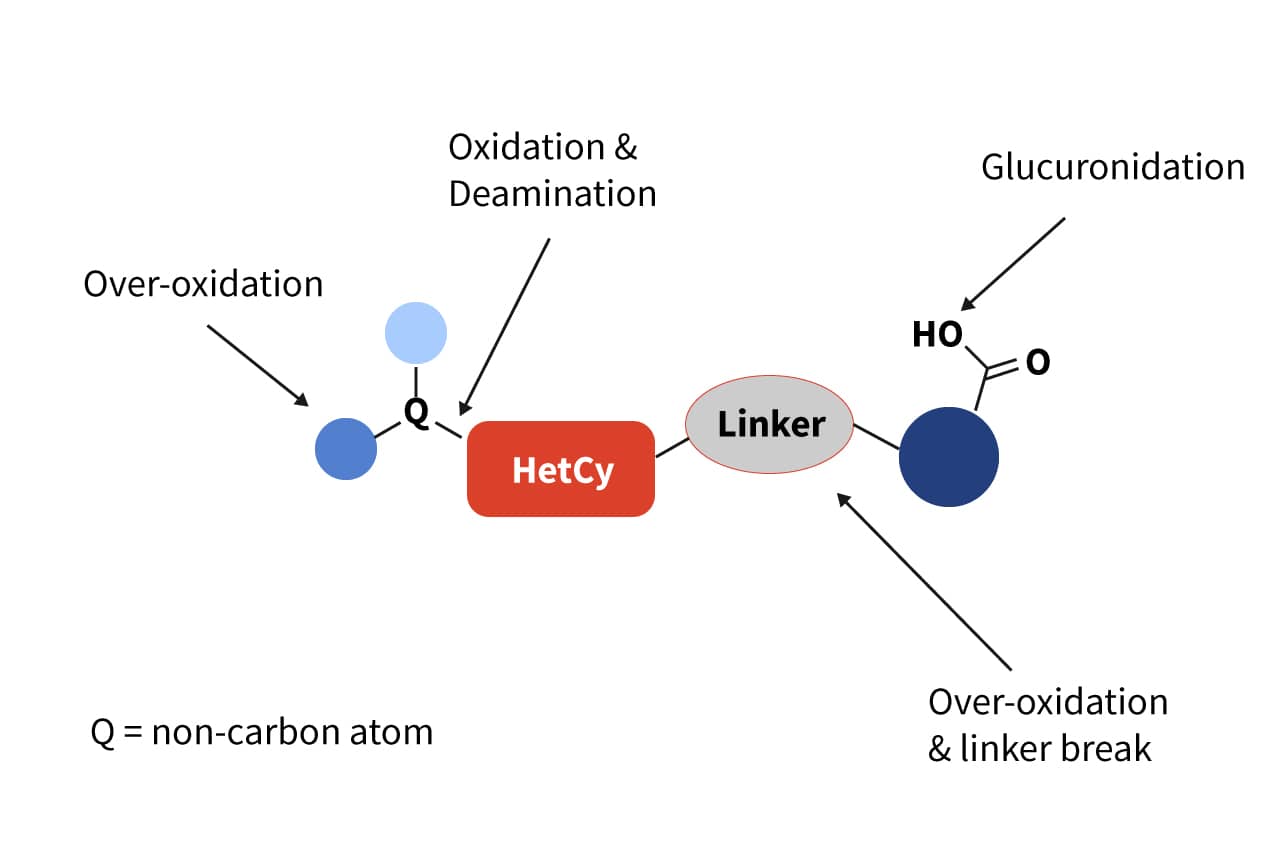
Figure 1: Liabilities shown in the lead series in the Met-ID studies.
We discovered that:
Metabolic Liabilities and Optimization
Metabolite identification (Met-ID) studies identified liabilities in different positions of the molecule (Figure 1) such as over-oxidation, deamination and glucuronidation. Strategies to improve metabolic stability (Figure 2) included:
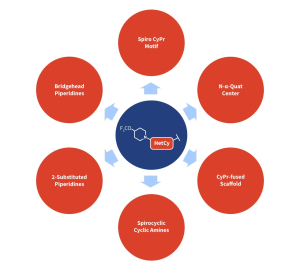
Figure 2: Structural modifications to improve in-vivo Clearance and/or Oral absorption.
Over 150 analogues were synthesized and evaluated, including hybrids that combined optimized HetCy and LHS amine features. Several compounds showed both strong cellular potency and favorable PK profiles. Ultimately, the team progressed four compounds through mouse efficacy studies, each of which demonstrated greater than 75% tumor growth inhibition (TGI). Additionally, one of these molecules advanced toward preclinical candidate nomination, marking a significant milestone in the program.
Aragen’s Integrated Drug Discovery (AIDD) is built to support your drug discovery journey with a seamless, end-to-end platform. Whether you’re advancing a small molecule or a protein degrader, our in-house capabilities—spanning synthetic chemistry, in vitro and in vivo biology, ADME, pharmacology, protein production, computational chemistry, and process development—are designed to accelerate your path from concept to candidate.
Our experienced scientists collaborate closely with you to tailor solutions that fit your target class and therapeutic area, including oncology, neuroscience, and cardiovascular diseases. With deep expertise and a flexible, integrated approach, we help you overcome discovery challenges and move promising candidates confidently toward IND-enabling studies.
