

Palatability is a critical factor in the development of oral veterinary formulations, especially for companion animals such as canines. Beyond therapeutic efficacy and stability, successful formulations must ensure voluntary intake and compliance during treatment.
Aragen developed a dual-API chewable tablet (API-A and API-B) for canine administration, across three dosage strengths. Both APIs exhibited intense bitterness and unpleasant mouthfeel, resulting in poor voluntary intake (~45% acceptance) and inconsistent dosing during in vivo palatability studies. The initial prototype also faced challenges with flavor–excipient incompatibility, moisture sensitivity leading to texture softening, and content uniformity issues.
To address these challenges, the team applied a systematic formulation design strategy—integrating taste masking, excipient compatibility screening, and process optimization—to create a stable, palatable, and manufacturable chewable tablet suitable for canine use.
The initial chewable tablet, intended for once-daily dosing in medium-sized canines, demonstrated therapeutic efficacy but poor sensory performance. The following key challenges were identified:
These factors collectively compromised the sensory profile, physical stability, and reproducibility of the final product, as summarized in Table 1.
Table 1. Key development challenges
| Parameter | Initial Observation | Target Specification | Challenge |
| Voluntary Intake (Canines, n=10) | 45% | ≥80% | Poor palatability due to bitterness |
| API Content Uniformity (%) | 80–112 | 95–105 | Inconsistent blend uniformity |
| Odor Profile | Unfavorable | Acceptable to canine | API bitterness dominated aroma |
| Storage Stability (40°C/75%RH, 3M) | 82% recovery (API – B) | 95–98% | Instability due to excipient–flavor incompatibility |
Aragen’s formulation development team employed an integrated development strategy involving taste masking, excipient compatibility testing, and process optimization.
1. Pre-formulation & Compatibility Screening
2. Palatability Optimization
A comprehensive palatability enhancement study was conducted to explore optimal combinations of natural flavor components (1 & 2) and synthetic flavor enhancers.
3. Composition and Process Optimization
The optimized chewable tablet (F10) demonstrated substantial improvements in palatability, uniformity, aroma masking, and stability (Table 2).
Table 2: Study outcomes
| Attribute | Baseline | Optimized (F10) | Improvement |
| Voluntary Intake (%) | 45 | 85 | +90 |
| API Content Uniformity (%) | 80–112 | 97–104 | Significantly improved |
| Odor Profile | Unfavorable | Acceptable | Effective aroma masking |
| Storage Stability (40°C/75% RH, 3M) | 82% recovery | 98% recovery | Enhanced formulation stability |
The formulation displayed excellent chewable texture (hardness 25–30 N) and mechanical integrity (friability <0.3%). Scale-up from 2 kg lab batches to 20 kg pilot batches at the client facility verified robust process reproducibility and blend homogeneity (RSD < 2%) with no flow or segregation issues. Figure 1 captures the palatability improvement in terms of canine voluntary intake studies.
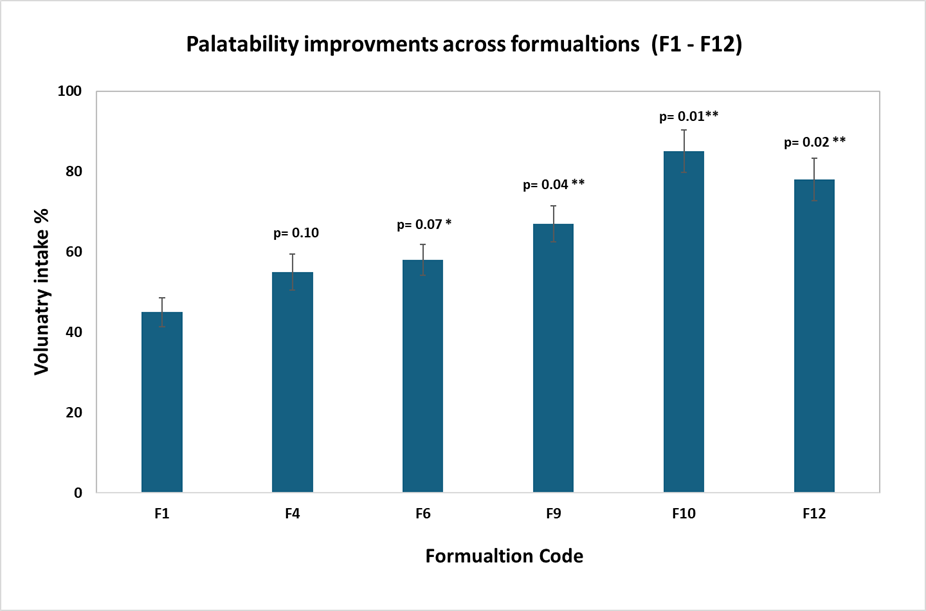
Figure 1. Comparative palatability improvements across formulations (F1–F12), with F10 achieving 85% voluntary intake. Bars represent mean values ± standard deviation. Asterisks indicate significance levels: p < 0.10 (*), p < 0.05 (**), p < 0.01 (***) vs F1.
The optimized chewable formulation:
Through a strategic blend of formulation design and sensory assessment, Aragen successfully developed a stable, scalable, and palatable dual-API chewable tablet for canine health. This achievement highlights Aragen’s expertise in creating innovative, animal-focused solutions that seamlessly integrate scientific precision with enhanced sensory acceptance.
At Aragen, we integrate pharmaceutical science, sensory design, and process excellence to develop animal health formulations from that combine efficacy with high acceptability. Our capabilities include:
Whether your goal is to improve product acceptance, enhance compliance, or strengthen formulation robustness, Aragen offers science-driven that accelerate the success of your animal health products—efficiently and effectively.
