
Proteins are universally composed of 20 canonical amino acids, each defined by triplet codons. While, these natural amino acids form the foundation of all biological systems, they offer a limited set of functional groups. In contrast, post-translational modifications, and the rare inclusion of two unnatural amino acids, namely selenocysteine and pyrrolysine, highlights nature’s own attempts to expand greater chemical diversity.
Unnatural amino acids (UAAs) serve as synthetic building blocks that surpass these natural limitations. By introducing new chemical functionalities, UAAs enable targeted improvements in protein stability, receptor selectivity, and pharmacokinetic properties—unlocking new potential for peptide and protein-based therapeutics. This growing significance is reflected in clinically approved UAA-containing drugs such as methyldopa, baclofen, gabapentin, bortezomib and sitagliptin.
The key facets of drug discovery and development include ADME optimization, structural motif identification, pathway targeting, structure-function refinement, and clinical drug advancement—areas where UAAs can be leveraged to enhance therapeutic design and efficacy.

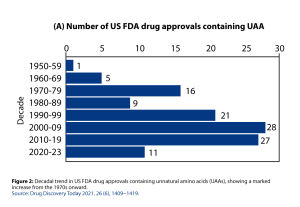
Driven by advances in synthetic techniques and the availability of structurally diverse UAAs, hundreds of drugs containing UAAs have been approved by the FDA over the past few decades. This trend reflects a marked increase in the utilization of UAAs beginning in the 1970s and continuing to expand in modern drug development (Figure 2). Many more UAA-containing drugs are currently in development or clinical trials.
Despite their immense potential, UAAs must be synthesized prior to use, presenting significant technical challenges including:
To overcome these hurdles, scalable and high-yield synthetic methodologies capable of producing UAAs with exceptional chiral purity and diverse functionality are essential.
Leveraging extensive expertise in organic synthesis and peptide chemistry, Aragen developed robust, reproducible routes for UAA production. Employing state-of-the-art synthetic techniques and rigorous optimization, the team consistently achieved greater than 99% 99% enantiomeric purity across various structural classes including functionalized UAAs such as (hetero)-aromatic, N-Methylated, beta-amino-acids. Key methodologies include:
1. Nickel complex-mediated UAA synthesis
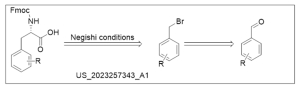
2. Negishi cross-coupling for UAA synthesis
3. N-methylated UAA synthesis using oxazolidinone-based chemistry
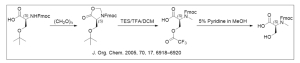
4. β-amino acid synthesis
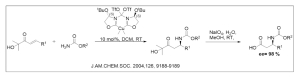
Different synthetic approaches to access UAAs often rely on chiral auxiliaries to enable enantioselective synthesis from achiral precursors such as propanoic acid (Figure 3).

Aragen synthesized a diverse library of UAAs such as aliphatic, aromatic, heteroaromatic, and β-amino acid derivatives, designed to improve peptide stability, bioavailability, and receptor interaction profiles.
These advancements have empowered clients to overcome key challenges by providing reliable access to unique UAAs, enabling accelerated timelines, enhanced molecular diversity, and robust data quality—while laying the groundwork for more sustainable UAA synthesis practices.
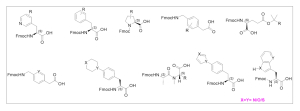
With over 20 years of organic synthesis expertise and nearly a decade of solid-phase peptide synthesis (SPPS) experience, Aragen partners with global biotech and pharmaceutical companies to provide:
Aragen transforms complex UAA synthesis challenges into opportunities for innovation, empowering clients to design next-generation peptide therapeutics with precision and confidence.
