
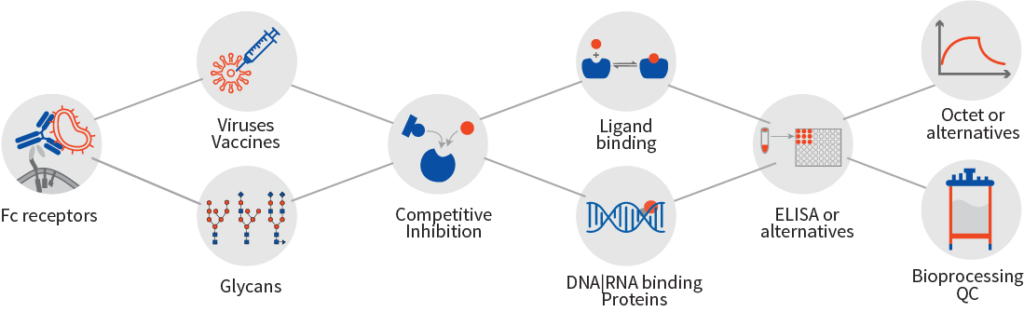
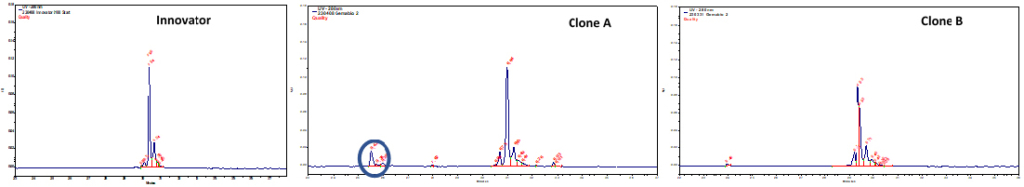
Incorporation of analytics at an early stage of cell line development accelerates right clone selection, leading to a significant reduction of the project timelines and resource requirements. Employing cIEF to eliminate the undesired basic peak clone (clone A) that do not overlay with the innovator.
| Protein Analytics | Quality Attribute | Platform |
|---|---|---|
| Protein concentration by A280 | Quantity | UV-Vis |
| A280, A320, Appearance | General- Appearance, Trubidity | |
| Free-thiol by Ellman’s assay | Primary Structure – Free thiol | |
| Enzyme kinetics (kcat,kcat/KM) | Potency | |
| N-Glycan profiling by CE | Primary Structure – PTMs | SCIEX PA 800 Plus |
| Charge variants by clEF | Purity – Charge variants | |
| Charge variants by CZE | Purity – Charge variants | |
| Size variants (fragments) by CE-SDS | Purity – Size variants |


| Protein Analytics | Quality Attribute | Platform |
|---|---|---|
| Slab gel IEF | Purity—Charge variants | Gel Electrophoresis |
| SDS-PAGE (non-reduced and reduced) | Purity —Size variants Identity | |
| Western blot | Identity | |
| Binding kinetics (kon,koff, KO) | Potency, Identity | BioLayer Interferometry (BLI) ForteBio Octet RED or Surface Plasmon Resonance (SPR) Biacore Molecular Decices SpectraMax |
| FcR and C10, binding | Half-life, Safety | |
| Protein concentration, antigen binding titer | Quantity | |
| Epitope binning | Epitope characterization | |
| Functional blocking | Potency Identity | |
| Yes/No binding | Identity | |
| Binding ELISA | Potency | |
| HCP ELISA (Cygnus) | Safety | |
| Residual Protein A/G/L by ELISA | Safety | |
| Endotoxin by Limulus Amebocyte Lysate (LAL) | Safety | Charles River EndoSafe Culturing |
| Bioburden, Sterility Test | Safety |