

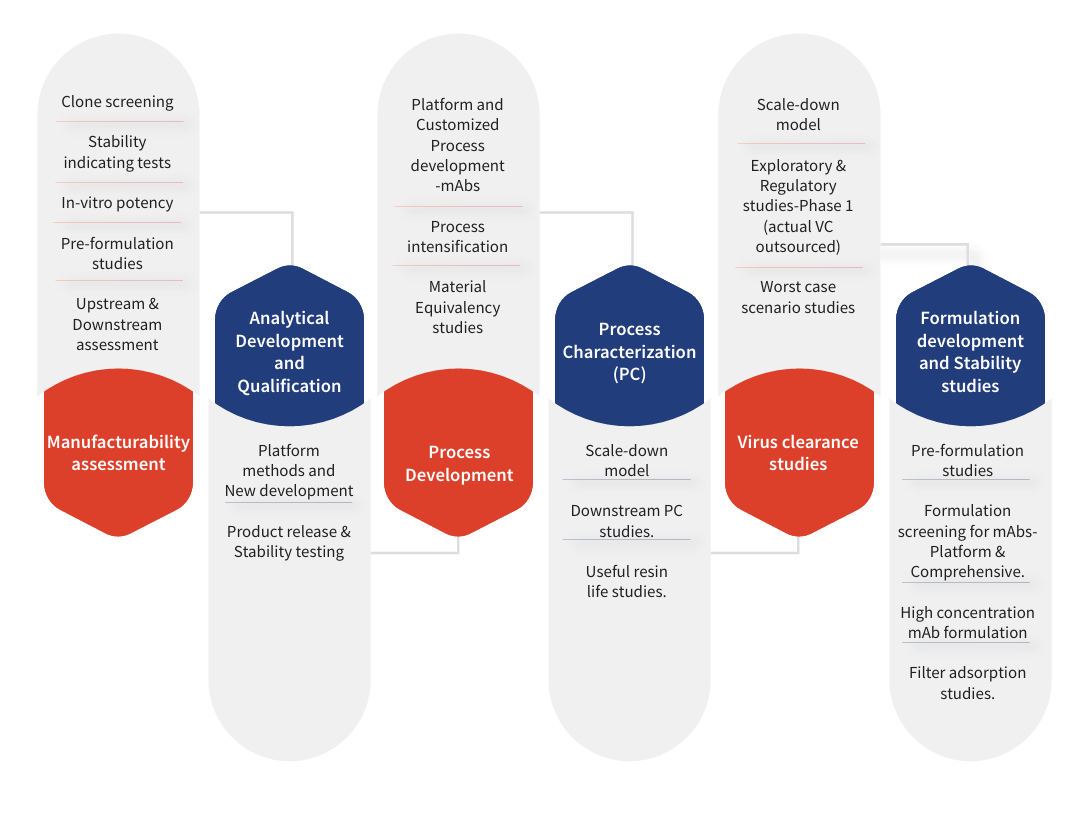
Clone screening
Stability
indicating tests
In-vitro potency
Pre-formulation
studies
Upstream &
Downstream
assessment
Platform
methods and
New development
Product release &
Stability testing
Platform and
Customized
Process
development
-mAbs
Process
intensification
Material
Equivalency
studies
Scale-down
model
Downstream PC
studies
Useful resin
life studies
Scale-down
model
Exploratory &
Regulatory
studies-Phase 1
(actual VC
outsourced)
Worst case
scenario studies
Pre-formulation
studies
Formulation
screening for mAbsPlatform &
Comprehensive
High concentration
mAb formulation
Filter adsorption
studies
