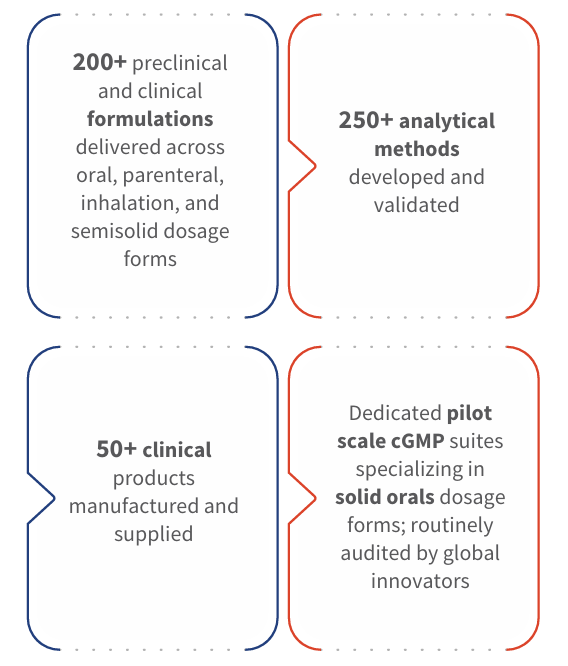
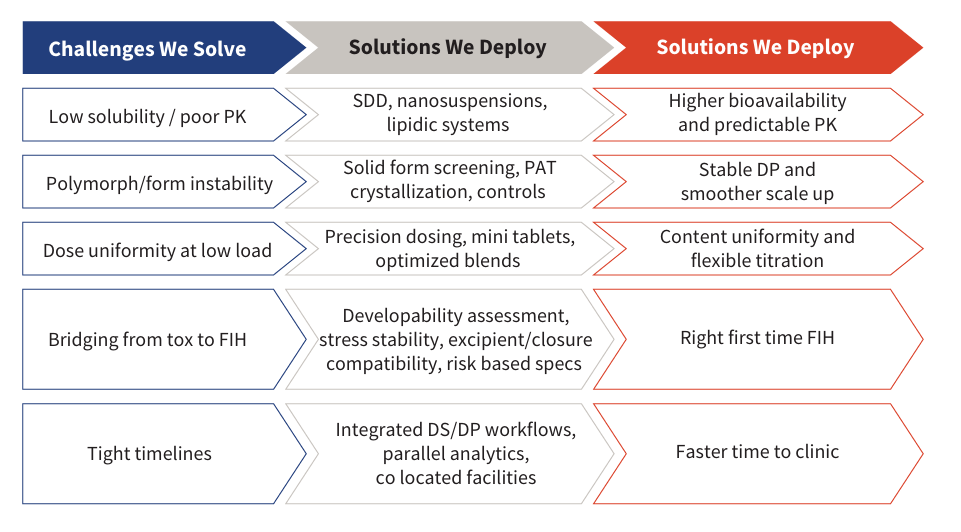
Accelerating your molecule from discovery to clinic, our integrated formulation services support everything from preformulation to FIH (First-in-Human) and GMP clinical manufacturing. With co-located teams and deep NCE expertise, we deliver clinic-ready drug products with speed, science, and scale.