

High-throughput electrophysiology (HTE) has emerged as a pivotal technique in drug screening and discovery, particularly for ion channel targets. Ion channels are fundamental to cellular function, regulating the flow of ions such as sodium, potassium, calcium, and chloride across membranes. These proteins play critical roles in processes including muscle contraction, neurotransmission, and cardiac rhythm. Dysfunctions in ion channels—whether due to genetic mutations or disease states—are implicated in a wide range of disorders such as epilepsy, cardiac arrhythmias, chronic pain, and cystic fibrosis. Consequently, ion channels are amongst the most important target classes in drug discovery.
Within the pharmaceutical industry, ion channels rank as one of the largest classes of protein targets, second only to G protein-coupled receptors (GPCRs) and kinases. Both voltage-gated and ligand-gated ion channels are actively pursued, with numerous drug candidates progressing through preclinical and clinical drug development stages. High-throughput electrophysiology techniques enable the efficient and rapid screening of compounds targeting these ion channels, accelerating the identification and optimization of potential therapeutic agents.
Electrophysiology techniques enable direct measurement of electrical activity in cells, tissues, and organs by probing ion channel function. Traditional patch-clamp electrophysiology provides high-resolution, real-time data on ion channel kinetics, gating mechanisms, and drug interactions. However, manual patch clamp, even though gold standard is labor-intensive and low throughput, and requires extensive expertise driven experience limiting its application in large-scale drug screening.
The emergence of high-throughput electrophysiology platforms has transformed this landscape. Automated planar patch-clamp systems-classified as HTE techniques-utilize microfabricated chips with arrays of apertures that capture and seal cells automatically (Figure 1), enabling simultaneous recordings from multiple cells. This architecture dramatically increases sample throughput while maintaining the precision and temporal resolution of traditional patch-clamp.
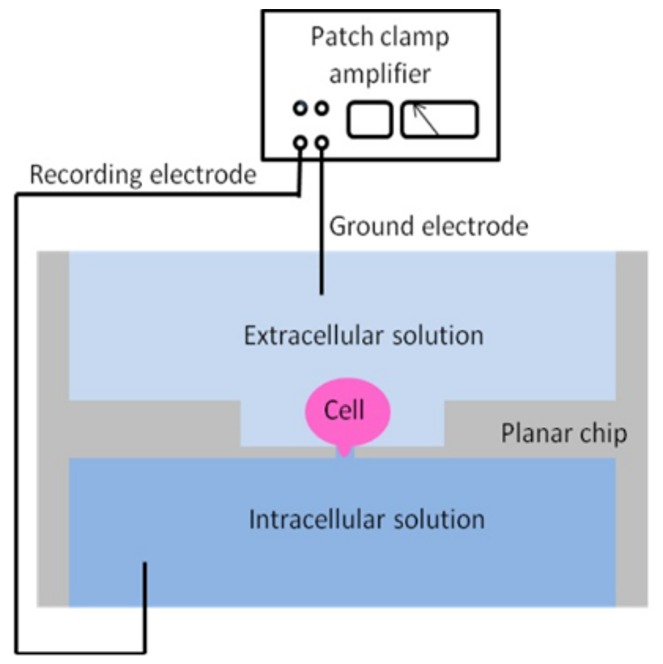
Automation reduces operator variability and enhances reproducibility, allowing parallel testing of multiple compounds across diverse ion channel targets. Moreover, these systems accommodate a wide range of cell types—including primary cells and engineered lines—broadening their utility in drug discovery pipelines. As the industry moves toward personalized medicine and targeted therapies, electrophysiology’s role in elucidating mechanisms of action and optimizing drug efficacy and safety continues to expand.
Accelerated Lead Identification and Validation
HTE platforms enable rapid screening of large compound libraries against ion channel targets. By automating patch-clamp recordings, researchers can evaluate hundreds of compounds efficiently, reducing time and cost compared to manual methods. This capability has been instrumental in identifying selective modulators that balance efficacy with safety. For instance, selective blockers of the NaV 1.7 sodium channel have been identified using HTE to address chronic pain, with careful avoidance of off-target effects on cardiac NaV 1.5 channels, which can cause arrhythmias (Swain et al., 2017).
Similarly, HTE contributed to the development of ivacaftor, a potentiator of the cystic fibrosis transmembrane conductance regulator (CFTR), accelerating its clinical development. These examples underscore HTE’s pivotal role in optimizing compound selectivity and safety early in drug development (Smyth 2020).
Neurological Disorder Drug Discovery
Many neurological diseases involve dysregulated ion channel activity. High-throughput electrophysiology enables efficient screening of compounds targeting voltage-gated sodium and calcium channels implicated in epilepsy, neuropathic pain, and migraine. Additionally, HTE supports investigation of other pharmacologically relevant channels such as GABA(A) and nicotinic acetylcholine receptors for epilepsy, NMDA receptors for Alzheimer’s, and TRP channels involved in neurological disorders, cancer, and heart disease.
Cardiac Safety Assessment
Cardiotoxicity is a leading cause of drug attrition in preclinical and clinical development. To address this, the Comprehensive in vitro Proarrhythmia Assay (CiPA)—a nonclinical safety pharmacology framework designed to identify electrophysiological mechanisms that may cause arrhythmia, uses HTE to evaluate the potential drug effects on cardiac ion channels, especially the hERG potassium channel critical for cardiac repolarization (Sager et al., 2014). Early HTE screening under the CiPA paradigm helps identify compounds with proarrhythmic risk, supporting safer drug development and regulatory compliance.
Mechanistic Studies and Pharmacodynamics
Understanding a drug’s mechanism of action (MoA) is essential for optimizing efficacy and minimizing side effects. HTE provides detailed, real-time data on voltage- and time-dependent interactions of drugs with ion channels. For example, epilepsy drugs such as lacosamide have been characterized using HTE to define their selective inhibitory effects on sodium channel excitability, informing dose optimization and therapeutic windows.
At Aragen, we offer advanced automated patch-clamp electrophysiology services designed to accelerate ion channel drug discovery and cardiac safety profiling. By integrating the QPatch 48X platform (Sophion Bioscience) into our workflows, we enable high-throughput, parallel analysis of up to 48 cells, allowing for efficient screening, in-depth pharmacological characterization, with industry-compliant data quality and consistency.
A key specialization of our electrophysiology team is cardiac risk evaluation through the hERG assay, a globally recognized benchmark for assessing proarrhythmic potential. We employ hERG potassium channel expressing stable cell lines and a validated panel of reference compounds (E-4031, verapamil) and test compounds.
The platform technology combines real-time data acquisition, automated cell handling, and expert analysis to deliver reproducible, high-fidelity data—minimizing variability and supporting confident decision-making. Aragen leverages state-of-the-art technology combined with expert analysis, positioning itself as a leading partner for ion channel research.
