

Cell line development (CLD) is a foundational process in the manufacture of biologics, including monoclonal antibodies and recombinant proteins. Each step in the CLD workflow (Figure 1) presents unique scientific and operational challenges. Addressing these challenges through evidence-based strategies is essential for Contract Research, Development, and Manufacturing Organizations (CRDMOs) to deliver robust, scalable, and high-quality biologics. This article explores the major challenges encountered at each step of the CLD process and presents scientifically validated
solutions that CRDMOs employ to address them.
The selection of an appropriate host cell line is foundational to CLD success. Chinese Hamster Ovary (CHO) cells are the predominant choice due to their capacity for human-like post-translational modifications, including glycosylation, essential for therapeutic effectiveness and safety. However, variability among CHO subclones and engineered variants
affects growth, metabolism, and protein secretion efficiency.
Challenges:
Solutions:
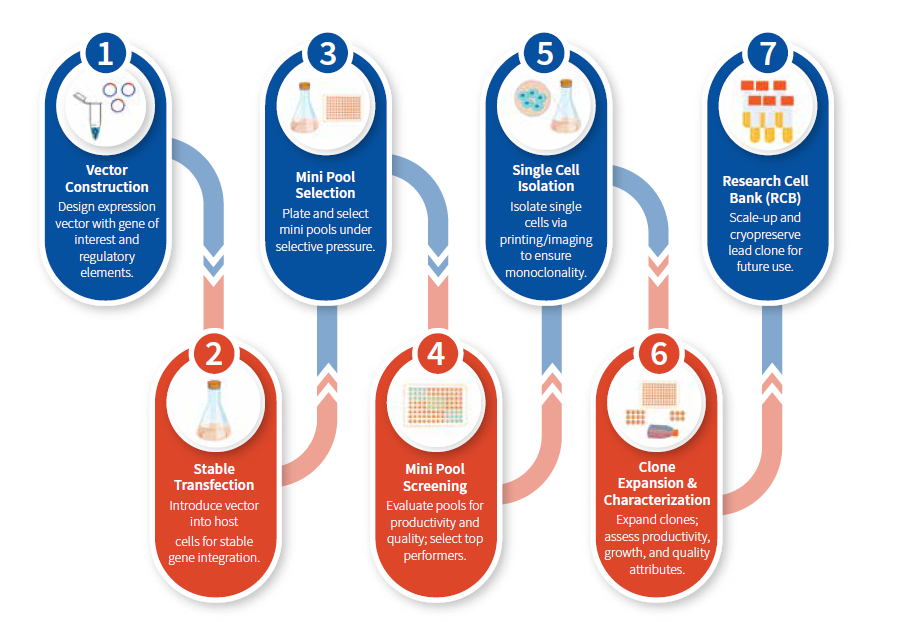
Figure 1: Cell Line Development (CLD) process flow.
Vector design influences transgene expression. Strong promoters, enhancer elements, and chromatin-opening
sequences are crucial for high, stable expression.
Challenges:
Solutions:
Following stable transfection, cells are plated as mini pools (MPs)—small groups of clonally related cells—to enrich for populations that stably express the transgene at high levels under selective pressure.
Challenges:
Solutions:
Screening MPs involves comprehensive evaluation of productivity, growth kinetics, and preliminary product quality to select the most promising candidates for single-cell cloning.
Challenges:
Solutions:
Isolation of single cells from selected MPs is essential to establish monoclonal populations—a regulatory
requirement—and to reduce heterogeneity..
Challenges:
Solutions:
Following isolation, clones are expanded under controlled conditions and subjected to rigorous evaluation of productivity, growth kinetics, and Critical Quality Attributes (CQAs).
Challenges:
Solutions:
Optimizing culture media formulation and bioprocess parameters is critical to enhance cell growth, viability, and protein expression.
Challenges:
Solutions:
Extended stability studies are essential to confirm that lead clones maintain productivity and product quality over multiple passages and extended culture periods.
Challenges:
Solutions:
The final step involves scaling up the lead clone and cryopreserving cells to establish a RCB that serves as the source for clinical and commercial manufacturing.
Challenges:
Solutions:
Cell line development is a complex and operationally demanding process that requires meticulous attention at every
stage. By integrating advanced genetic engineering, automated high-throughput screening, multi-attribute analytical
characterization, and systematic media and process optimization, CRDMOs can develop stable, high-producing cell
lines with consistent quality attributes. This workflow-centric, evidence-based approach accelerates development
timelines, reduces risk, and ensures scalable, regulatory-compliant biologics manufacturing.
