

High selectivity is essential to maximize desired product formation while minimizing impurities, lowering purification needs, solvent use, and costs. Achieving this level of selectivity can be challenging in traditional batch processes, which often suffer from uneven mixing and temperature control, leading to impurity formation and product degradation. Continuous flow processing addresses these limitations by providing precise residence times, rapid and uniform mixing, and efficient heat and mass transfer, effectively suppressing side reactions and preventing over-reaction. As a result, reactions in continuous flow exhibit more stable profiles, higher selectivity, and greater robustness, enabling reliable scale-up and commercial production.
A potassium tert-butoxide–mediated transformation operated in batch at 20–25 °C for ~30 minutes delivered only ~58% product (area %) with multiple impurities and instability on extended holds. The impurity profile—tracked by Relative Retention Time (RRT) values—showed several significant peaks alongside Stage Intermediates (SM and Product), underscoring downstream burden and poor scaleup fitness. This made the step unsuitable for largescale production and set the stage for a continuous flow feasibility and optimization program (see Table 1).
Table 1: Batch Reaction Profile (Baseline)
Abbreviations: RRT = Relative Retention Time; STG = Stage Intermediate.
| S.No. | Reactor | Temperature (°C) | RRT 0.91 | RRT 0.96 | Product (% area) | RRT 1.08 | RRT 1.18 | SM (% area) | RRT 1.27 | RRT 1.33 |
| 1 | Batch | 20–25 | 13.39 | 1.39 | 58.47 | 10.78 | 2.72 | 4.14 | 0.44 | 4.54 |
This study had the following objectives:
Feasibility in Microstructured Flow
Feasibility and early optimization were performed in an Advanced Flow Reactor (AFR; G1) equipped with two heart-shaped plates (2 × 2.7 mL; total internal volume 5.4 mL). The initial trial demonstrated product formation with a profile similar to batch at a Residence Time (RT) of 4.7 minutes (Figure 1).

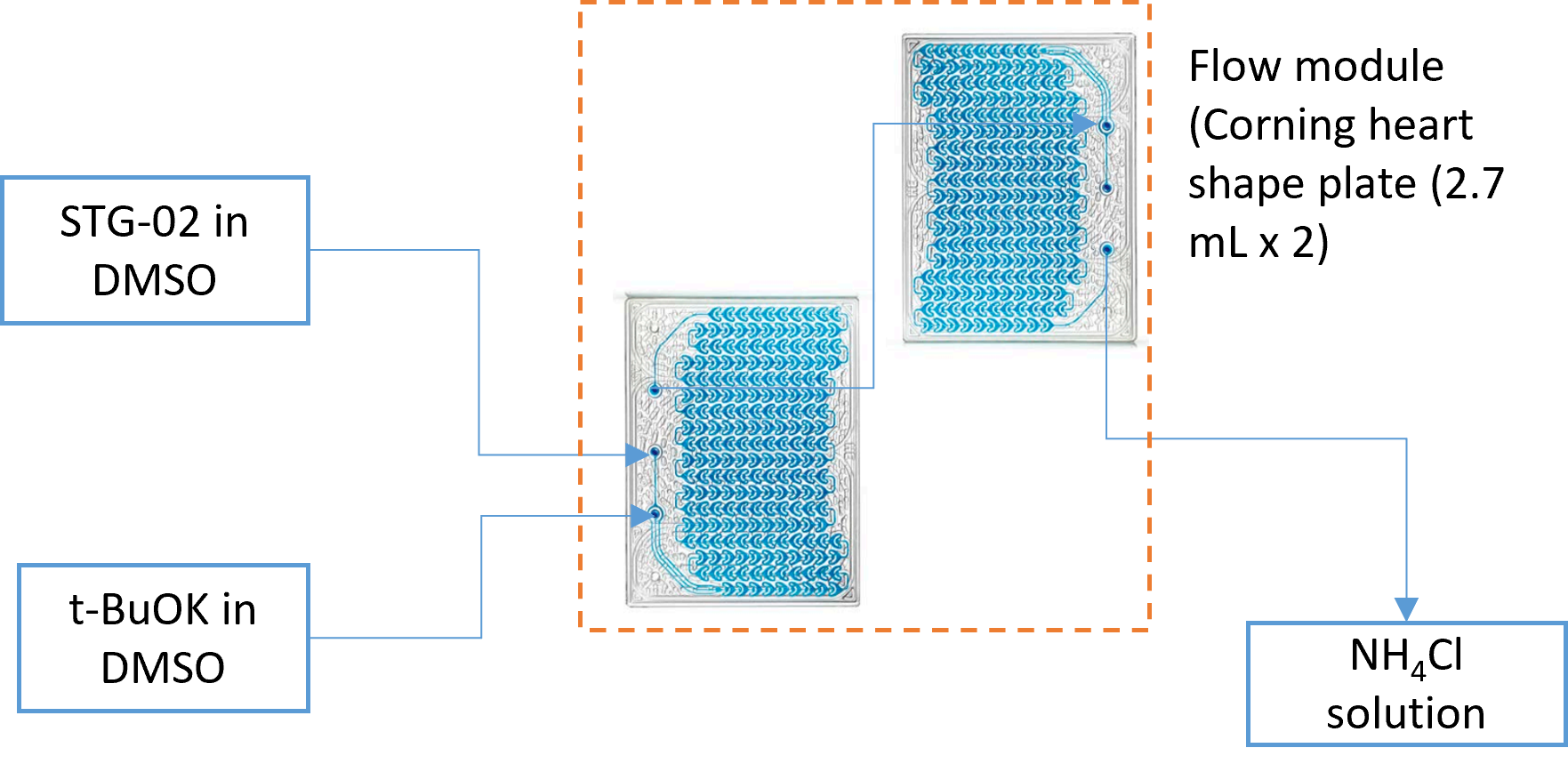
Figure 1: (A) Continuous flow setup with Advanced Flow Reactor (AFR) showing the pump/controls and reactor module used for the potassium tert-butoxide step. (B) Process schematic: Process schematic: Highlights efficient mixing, precise RT (Residence Time) control, and a clean quench—key to suppressing impurities and degradation versus batch. SM (Starting Material) and tBuOK (potassium tert-butoxide) feed the Advanced Flow Reactor) “heart” module (two plates in series), where rapid mixing at fixed RT drives conversion, followed by NH₄Cl quench to stabilize the product stream.
Optimization of Kinetics and Temperature
A targeted matrix varying RT (4.7–10.8 minutes) and temperature (25–35 °C) identified RT = 10.8 minutes at 30 °C as optimal, delivering ~75% product (area %) and reducing impurity levels versus batch. Comprehensive feasibility and optimization data are presented in Table 1.
Table 1. Feasibility and Optimization Trials (AFR; G1)
Abbreviations: AFR = Advanced Flow Reactor; RT = Residence Time; RRT = Relative Retention Time; SM = Starting Material; ND = Not Detected; STG = Stage Intermediate.
| S. No. | Batch No. | Reactor | RT (min) | Temp (°C) | RRT 0.91 | RRT 0.96 | Product | RRT 1.08 | RRT 1.18 | SM | RRT 1.27 | RRT 1.33 |
| (% area) | ||||||||||||
| 1 | Trial–1 | AFR (5.4 mL) | 4.7 | 25 | 0.64 | 0.15 | 50.08 | 1.29 | 6.49 | 33.1 | 3.35 | 0.14 |
| 2 | Trial–2 | AFR (5.4 mL) | 5.1 | 35 | 0.59 | ND | 63.11 | 2.18 | 10.23 | 19.6 | 0.13 | 2.44 |
| 3 | Trial–3 | AFR (5.4 mL) | 8.4 | 30 | 0.60 | ND | 61.10 | 2.00 | 9.06 | 22.50 | 0.16 | 2.46 |
| 4 | Trial–4 | AFR (5.4 mL) | 10.8 | 30 | 1.22 | ND | 75.45 | 2.83 | — | 6.09 | 0.09 | 3.06 |
Reproducibility and Scale Transition
Optimized conditions were transferred to an inhouse Plug Flow Reactor (PFR; 55 mL) to confirm reproducibility at larger holdup. The process reproduced the improved selectivity, achieving ~79% product (area %) with a similarly cleaner impurity profile (see Table 2). The optimized process was implemented on a pilot‑scale continuous flow system to demonstrate reproducible scale‑up under manufacturing‑relevant conditions (Figure 2).
Table 2. Reproducibility at Scale (In-house PFR)
Abbreviations: PFR = Plug Flow Reactor; RT = Residence Time; RRT = Relative Retention Time; ND = Not Detected; SM = Starting Material.
| S. No. | Fraction | Reactor | RT (min) | Temp (°C) | RRT 0.91 | RRT 0.96 | Product | RRT 1.08 | RRT 1.1 | SM | RRT 1.27 | RRT 1.33 |
| (% area) | ||||||||||||
| 1 | Fraction 01 | PFR (55 mL) | 13.7 | 30 | — | — | 58.34 | — | 2.70 | — | 3.96 | — |
| 2 | Fraction 02 | 2.37 | ND | 79.28 | 3.07 | 9.73 | ND | 1.59 | 3.08 | |||
| 3 | Fraction 03 | 1.71 | ND | 60.46 | 3.56 | 13.7 | ND | 14.56 | 2.75 | |||

Figure 2: Pilot‑scale continuous flow setup enabling reproducible scale‑up of reactive chemistries.
Higher selectivity and cleaner profiles
Product (area %) improved from ~58% under batch operation to ~75–80% under optimized flow conditions, with a significant reduction in RRT tracked impurities, lowering downstream purification burden.
Stability and robustness
Fixed Residence Time (RT), rapid and uniform mixing, and superior heat/mass transfer prevented the degradation observed during batch holds, stabilizing the reaction profile.
Scalability demonstrated
Performance reproduced at 55 mL PFR holdup and successfully demonstrated at 25 g scale, further establishing suitability for technology transfer and commercial manufacturing.
Overall, this case study illustrates how transitioning from batch to continuous flow can unlock higher selectivity, improved stability, and commercially scalable performance for challenging base‑mediated transformations.
Aragen combines deep flow‑chemistry expertise with strong process‑engineering capabilities to deliver cleaner profiles, higher selectivity, and reliable scalability. We have extensive experience in scaling up diverse chemistry—including nitration, cyclization, LiHMDS reactions, BAST chemistry and n-BuLi on multigram scale. Our ability to rapidly optimize kinetics, control impurities, and seamlessly transition from AFR to PFR systems demonstrates how we turn challenging batch steps into robust, manufacturing‑ready continuous flow processes.
