
Membrane proteins are integral components of cellular architecture that mediate vital biological processes such as signal transduction, molecular transport, and cell–cell communication. They constitute over 30% of the proteome and serve as targets for more than half of all approved drugs, underscoring their immense biomedical and pharmacological significance. However, native isolation of membrane proteins is often challenging—due to their low abundance, structural instability outside the lipid bilayer, and complex post-translational modifications. As a result, recombinant expression systems have become indispensable for producing membrane proteins in sufficient quantities for biochemical, biophysical, and structural studies.
Despite this promise, recombinant production remains technically challenging. The hydrophobic nature of membrane proteins often leads to misfolding, aggregation, or toxicity in host cells, while their extraction and purification require careful optimization of solubilization conditions using detergents or membrane mimetics. Addressing these challenges necessitates a multidisciplinary approach encompassing molecular biology, host engineering, and advanced purification strategies to ensure the recovery of functional, structurally intact membrane proteins.
Building on over a decade of experience in recombinant protein production and analytical development, Aragen has developed specialized capabilities in expressing and purifying challenging membrane proteins. Through a combination of rational construct design, host system optimization, and advanced solubilization strategies, Aragen effectively addresses the inherent challenges associated with membrane protein expression, including low yields, host toxicity, and misfolding.
This case study involves the recombinant production of three membrane protein classes, demonstrating Aragen’s expertise in selecting suitable expression systems and optimizing detergent extraction to deliver reliable, scalable, and high-quality solutions tailored to client needs.
Recombinant membrane proteins were expressed in host systems chosen according to protein origin and characteristics:
(See Figure 1 for representative structures of these membrane proteins.)
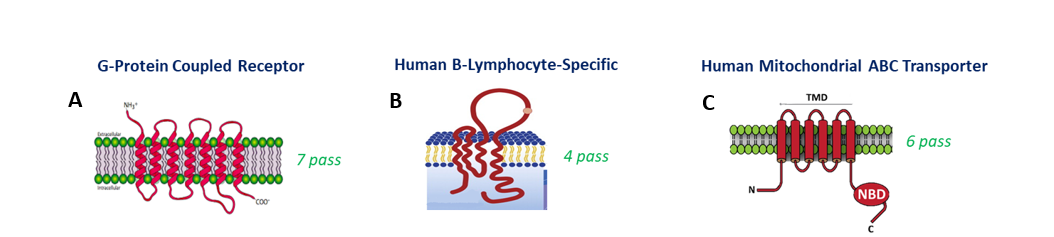
Figure 1: Representative structures of the recombinant membrane proteins produced in this study. (A) G-Protein Coupled Receptor (GPCR) highlighting seven transmembrane helices. (B) Human B-lymphocyte-specific membrane protein with extracellular and transmembrane domains. (C) Human mitochondrial ABC transporter showing nucleotide-binding and transmembrane domains. These structures illustrate the diversity and complexity of membrane proteins successfully expressed and purified using optimized workflows.
Cultures were scaled to 2–5 L, and cells were harvested and lysed to isolate membrane fractions, which were subsequently solubilized using mild detergents to preserve protein structure and functionality.
Protein purification was performed through a multistep workflow. Solubilized proteins were first purified using Ni-NTA affinity chromatography. For proteins requiring tag removal, such as mitochondrial ABC transporters, TEV protease digestion was performed, followed by subtractive Ni-NTA purification to remove uncleaved protein and protease. Finally, gel filtration chromatography was applied to remove aggregates and ensure monodispersity of the purified proteins.
Recombinant membrane proteins were successfully expressed and purified from their respective host systems. SDS-PAGE and Western blot analyses confirmed correct molecular weights and His-tag presence, while analytical size-exclusion chromatography (SEC) demonstrated protein homogeneity and stability where applicable, indicating suitability for downstream structural or functional studies.
1. GPCR (Sf9 Insect Cells)
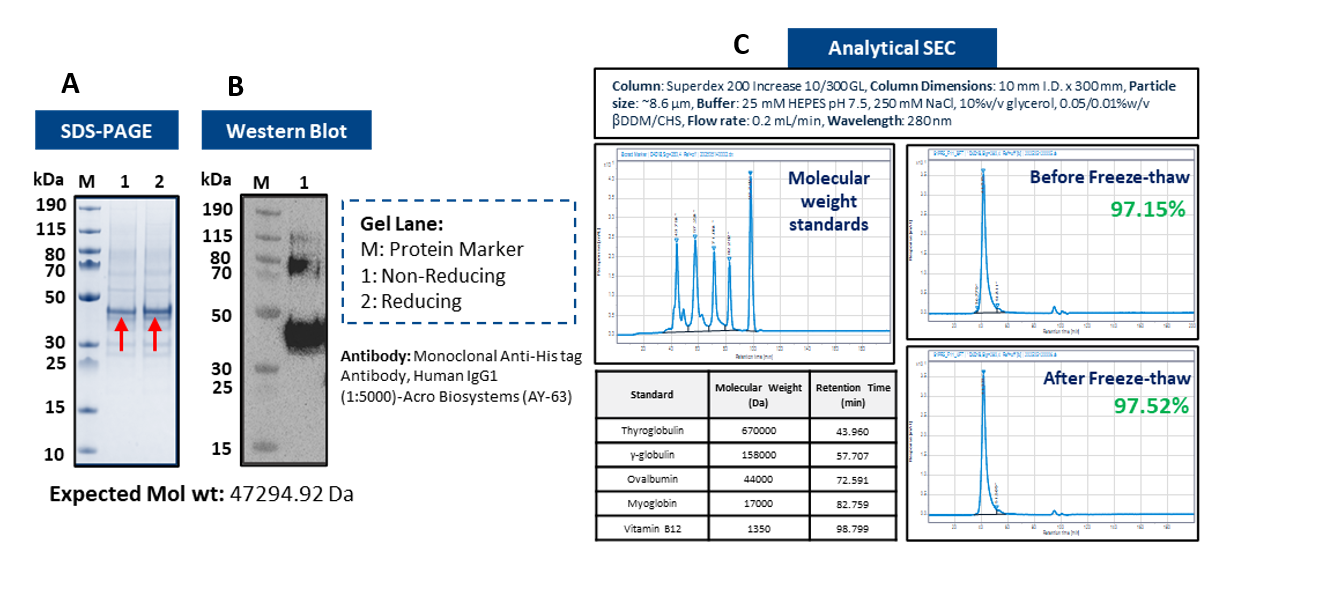
Figure 2: Recombinant GPCR from Sf9 cells: (A) SDS–PAGE (~50 kDa); (B) Western blot confirms His-tagged protein; (C) SEC >97 % purity and stability.
2. Human B-Lymphocyte-Specific Membrane Protein (Expi293 Cells)
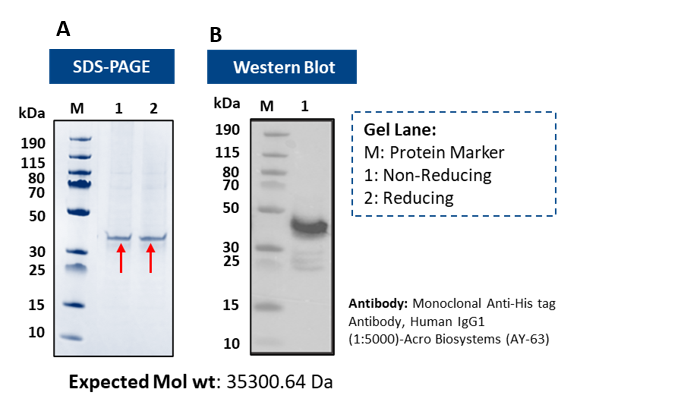
Figure 3: B-lymphocyte membrane protein from Expi293 cells: (A) SDS–PAGE (~35 kDa); (B) Western blot confirms His-tagged protein.
3. Human Mitochondrial ABC Transporter (E. coli BL21Star (DE3))
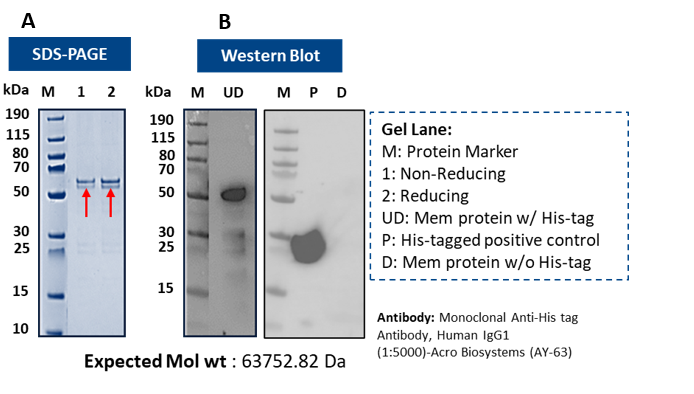
Figure 4: Mitochondrial ABC transporter from E. coli BL21 Star (DE3): (A) SDS–PAGE (~63–65 kDa); (B) Western blot verifies complete removal of His-tag by protease digestion.
These results demonstrate the effectiveness of the optimized workflows for recombinant membrane protein production designed by Aragen’s expert team. Tailoring expression systems and purification strategies to each protein enabled the generation of high-purity, correctly folded, and stable membrane proteins, suitable for structural biology, biophysical characterization, and early-stage drug discovery applications.
Aragen’s Cell & Protein Science team brings 10+ years of experience and depth of expertise in recombinant membrane protein production and analytical processes. We offer:
Unlock breakthroughs with Aragen! Our agile solutions empower your R&D—speed, flexibility, tailored precision—delivering high value as your trusted discovery biology services partner.
