
Pseudoproline and Dmb (2,4-dimethoxybenzyl) dipeptides play a critical role in peptide synthesis by overcoming aggregation and improving yield. They achieve this by disrupting secondary structures like β-sheets, which often hinder coupling efficiency.
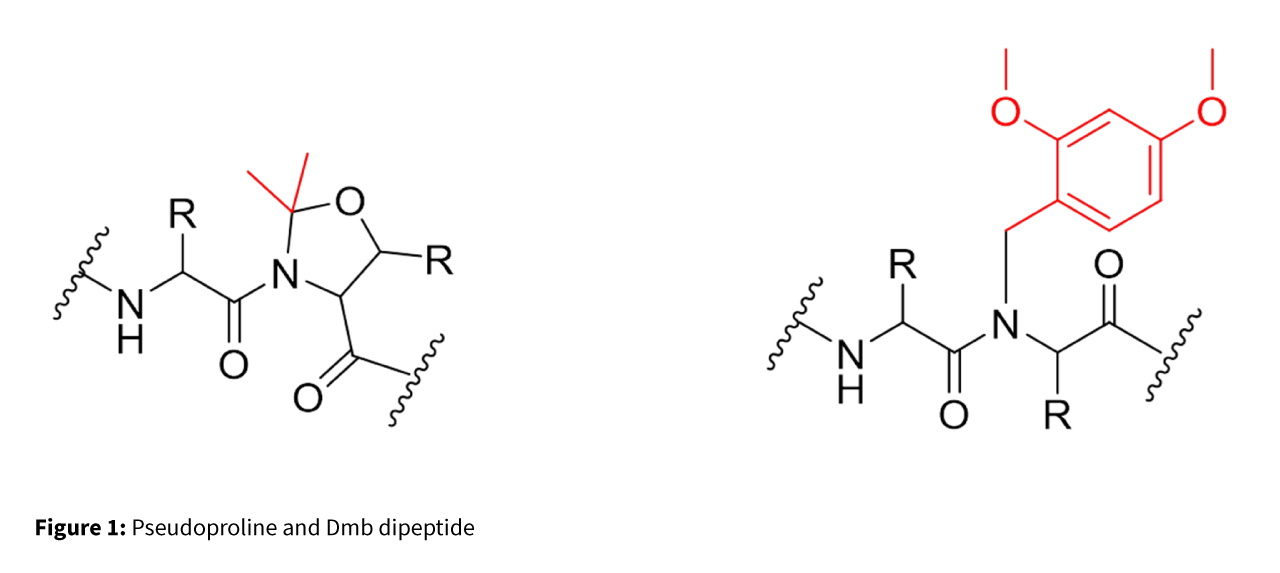
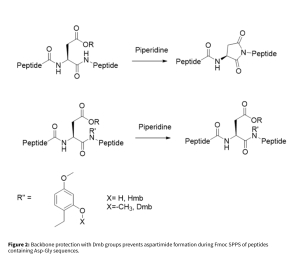
Both are incorporated as preformed dipeptides to improve coupling efficiency, solubility, and overall synthesis outcomes. Their ability to disrupt secondary structure—by exploiting the natural propensity of N-alkyl amino acids to prevent secondary structure formation during peptide assembly—leads to more predictable acylation and deprotection kinetics, faster reaction rates, and improved crude product quality in Fmoc SPPS. While powerful, each approach has limitations: pseudoproline dipeptides are restricted to Ser/Thr positions, and amino acid coupling after a Dmb residue can be challenging. To address these issues, Fmoc-Aaa-(Dmb)Gly-OH dipeptides are increasingly used for difficult hydrophobic peptides. Additionally, the N-Dmb backbone helps prevent aspartimide formation during Fmoc SPPS of peptides containing the Asp-Gly sequence (Figure 2).
This case study highlights how Aragen developed scalable, high-purity synthetic routes for Pseudoproline and Dmb dipeptides to help clients overcome peptide synthesis challenges and achieve improved yields and purity.
Despite their immense potential, these dipeptides must be synthesized in advance, presenting significant technical challenges including:
These challenges demand optimized synthetic strategies that ensure reliable access to pseudoproline and Dmb dipeptides with exceptional chiral purity, diverse functionality, and scalability—critical for enabling complex peptide synthesis.
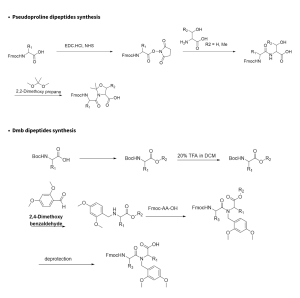
Our experts at Aragen applied their extensive expertise in organic synthesis and peptide chemistry to design optimized, reproducible routes for producing pseudoproline and Dmb dipeptides production for solid phase peptide synthesis. Employing state-of-the-art synthetic techniques and rigorous optimization, the team consistently achieved greater than 99% HPLC and enantiomeric purity across various functionalized pseudoproline and Dmb dipeptides including D-dipeptides, D/L-dipeptides and dipeptides incorporating unnatural amino acids etc.
General synthetic procedures include:
Aragen synthesized a diverse library of pseudoproline and Dmb dipeptides—including versions containing D- or unnatural amino acids—Aragen helps customers significantly improve crude purity and overcome bottlenecks in assembling challenging peptides.
The availability of these dipeptides has enabled customers to:
These outcomes demonstrate how reliable dipeptide availability translates into faster progress, higher purity, and reduced technical risk for customer programs.
With more than 20 years of organic synthesis expertise and over a decade of solid-phase peptide synthesis (SPPS) experience, Aragen partners with global biotech and pharmaceutical companies to provide:
Ready to overcome peptide synthesis challenges? Aragen transforms complex peptide synthesis challenges into opportunities for innovation—empowering you to design next-generation peptide therapeutics with precision and confidence.
Contact us today to discuss your project needs.
