

Co-crystals offer an innovative solution by enhancing key physicochemical properties—such as solubility, dissolution rate, and stability—while preserving the neutral state of the API. This is achieved through non-covalent interactions between the API and a co-former, which create a new crystal lattice with modified packing density and reduced lattice energy. These changes enhance solubility and dissolution without chemical modification, ensuring the therapeutic identity remains intact and regulatory compliance under the “same active” principle.
The need for such innovation stems from a persistent challenge in drug development: poor aqueous solubility, which often limits API bioavailability and therapeutic performance. Conventional approaches like salt formation or amorphous dispersions may not always be feasible, as they can require ionizable groups or alter the API’s molecular structure—potentially impacting its pharmacological profile.
This case study demonstrates how Aragen applied co-crystal technology to overcome solvent-related challenges and deliver a robust, scalable crystallization process that achieved high purity and consistency for the client.
The initial feasibility process for the co-crystal relied on Tetrahydrofuran (THF), a Class 2 solvent, as the solvent, but residual THF levels exceeded ICH limits, creating a regulatory concern. This made scale-up difficult, as controlling THF within acceptable limits was not practical. The challenge was to develop an alternative crystallization process that could deliver the desired polymorph with high yield and purity, while ensuring solvent compliance and scalability.
The Particle Science & Engineering team at Aragen executed a systematic, data‑driven crystallization development program to replace THF and deliver a robust, scalable process. The workflow comprised: (i) solvent landscape determination for the free base and the co‑crystal, (ii) MSZW (Metastable Zone Width) estimation in the selected solvent system using Crystal‑16, (iii) feasibility trials, (iv) process optimization, and (v) scale‑up.
1. Solvent Landscape & Mixed Solvent Screening
Equilibrium solubility was measured across almost 30 solvents at different temperatures. The free base was insoluble in most solvents; methanol, ethanol, and THF showed relatively higher solubility (THF later excluded due to ICH limits). To broaden options for crystallization, mixed solvent systems were screened to reach ~100 mg/mL solubility at 50–60 °C (Table 1).
Table 1: Solvent mixture screen (approximate solubility at 60 °C).
| S. No. | Solvent system | Temperature (oC) | Solubility (mg/mL) |
| 1 | 10% DMSO in Iso propyl acetate | 60 | 180 |
| 2 | MeOH/n-butyl acetate 65:35 | 60 | 50 |
| 3 | 10% DMSO in Toluene | 60 | 90 |
| 4 | IPA/2-MeTHF 40:60 | 60 | 65 |
| 5 | 10% DMSO in MTBE | 60 | 85 |
The above screen indicated DMSO/Iso propyl Acetate (IPAc, DMSO/Toluene, and IPA/2MeTHF as promising solvent systems for crystallization trials.
2. Feasibility Trials
Feasibility crystallizations were performed in DMSO/IPAc, DMSO/Toluene, and IPA/2-MeTHF:
Based on the feasibility experimental data, DMSO/IPAc solvent system was chosen for crystallization process optimization.
3. DMSO/IPAc Composition Optimization
To understand the recovery from DMSO/IPAc solvent system and design the crystallization process, different DMSO/IPAc mixtures were screened to optimize the DMSO composition (Table 2).
Table 2: DMSO/IPAc composition screen (70 °C).
| S. No. | Solvent system | Temperature (oC) | Solubility (mg/mL) | Solvent volumes |
| 1 | 5% DMSO in IPAc | 70 | 170 | 5.8 |
| 2 | 7% DMSO in IPAc | 70 | 210 | 4.7 |
| 3 | 10% DMSO in IPAc | 70 | 275 | 3.6 |
Follow-up thermodynamic solubility. Cocrystal solubility was then measured at 65 °C and 5 °C in 5% and 7% DMSO/IPAc, and at 5 °C in 1%, 2%, and 3% DMSO/IPAc to finalize isolation conditions:
4. MSZW Analysis
MSZW data were generated for the co‑crystal in 5% DMSO/IPAc using Crystal-16 (Table 3) to define the saturation profile and identify the seeding point.
Table 3: MSZW data (5% DMSO/IPAc)—Clear & Cloud points.
| Clear Point | Cloud Point | |
| Average | Average | |
| 125 | 65.6 | 45 |
| 100 | 63.5 | 44.9 |
| 83.3 | 55.4 | 30.65 |
| 71.43 | 52.5 | 28.4 |
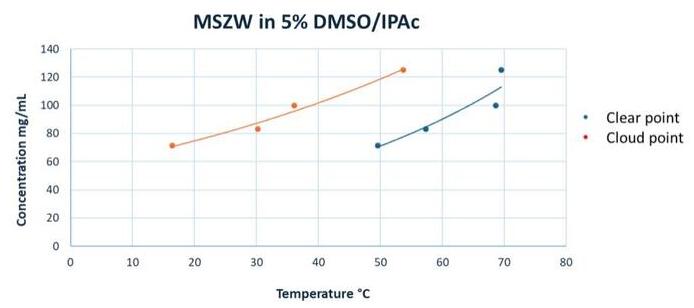
Figure 1 : MSZW profile (5% DMSO/IPAc). The broad metastable zone between the clear point (red) and cloud point (blue) curves is significant across the concentration range.
The above data (Figure 1) suggested that the broad metastable zone width (MSZW) observed in 5% DMSO/IPAc enables controlled nucleation through a seeding strategy at a defined temperature, rather than relying on spontaneous nucleation. This approach ensures a robust crystallization process with consistent particle size and reliable polymorph control.
5. Process Implementation & Scale-up
As inferred from the above results, optimization was executed in cylindrical reactors (HEL Polyblock and automated lab reactor). A seeding-based crystallization in the DMSO/IPAc system delivered a robust, reproducible process suitable for scaleup.
This work demonstrates how a systematic, data-driven approach—combining solubility profiling, mixed solvent screening, MSZW analysis, and controlled seeding—can overcome solvent-related challenges and deliver a robust, scalable co-crystal crystallization process. The optimized method achieved high yield, exceptional polymorph purity, and full compliance with regulatory solvent limits, ensuring process reliability for scale-up.
At Aragen, we apply advanced, data-driven crystallization strategies to design robust processes that ensure stability, performance, and scalability for drug substances. Our expertise spans from early development to commercial scale-up, delivering scientifically optimized solutions for complex challenges. Our capabilities include:
We combine science and technology to deliver robust, high-quality crystallization solutions—replacing problematic solvents, achieving consistent polymorph control, and enabling smooth scale-up with confidence.
Ready to optimize your crystallization process? Connect with us today to explore how Aragen can accelerate your development.
