

Crystallization is a critical unit operation in the pharmaceutical industry, essential for the purification and isolation of active pharmaceutical ingredients (APIs). However, the process is often complicated by phenomena such as “oiling out”, also known as liquid–liquid phase separation (LLPS). Oiling out occurs when the solution’s supersaturation—the driving force for crystallization—is not properly controlled, resulting in the formation of a secondary, solute-rich liquid phase rather than direct nucleation of a solid crystalline phase. This secondary phase can entrap impurities, hinder crystal growth, and disrupt downstream operations like filtration and drying, ultimately impacting product purity, yield, and process scalability.
At Aragen, the Particle Science & Engineering team encountered significant oiling out during the scale-up of an evaporative crystallization process. The process involved distillation of aqueous solution containing target compound, followed by co-distillation with isopropanol (IPA) to reduce water content and induce crystallization. However, rapid removal of water during co-distillation led to uncontrolled desupersaturation, triggering oiling out. This manifested as the appearance of a cloudy, heterogeneous suspension—a metastable emulsion of solute-rich droplets—rather than the desired crystalline solid. The oiling out phase caused:
To address the problem, the team employed Process Analytical Technology (PAT), specifically the Blaze Metrics probe, which enabled real-time, in-situ monitoring of the crystallization process via high dynamic range (HDR) imaging. This allowed for early detection and characterization of oiling out events, distinguishing between true solid–liquid suspensions and metastable liquid–liquid emulsions (Figure 1).

Key process modifications included:
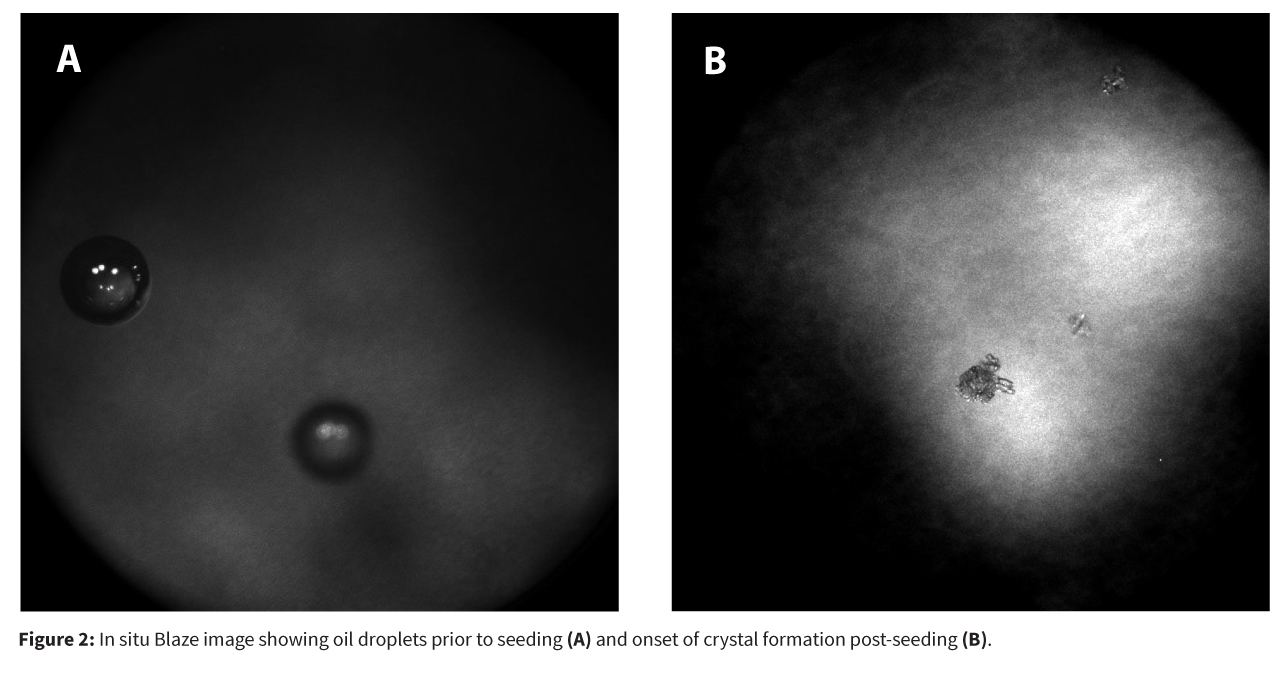
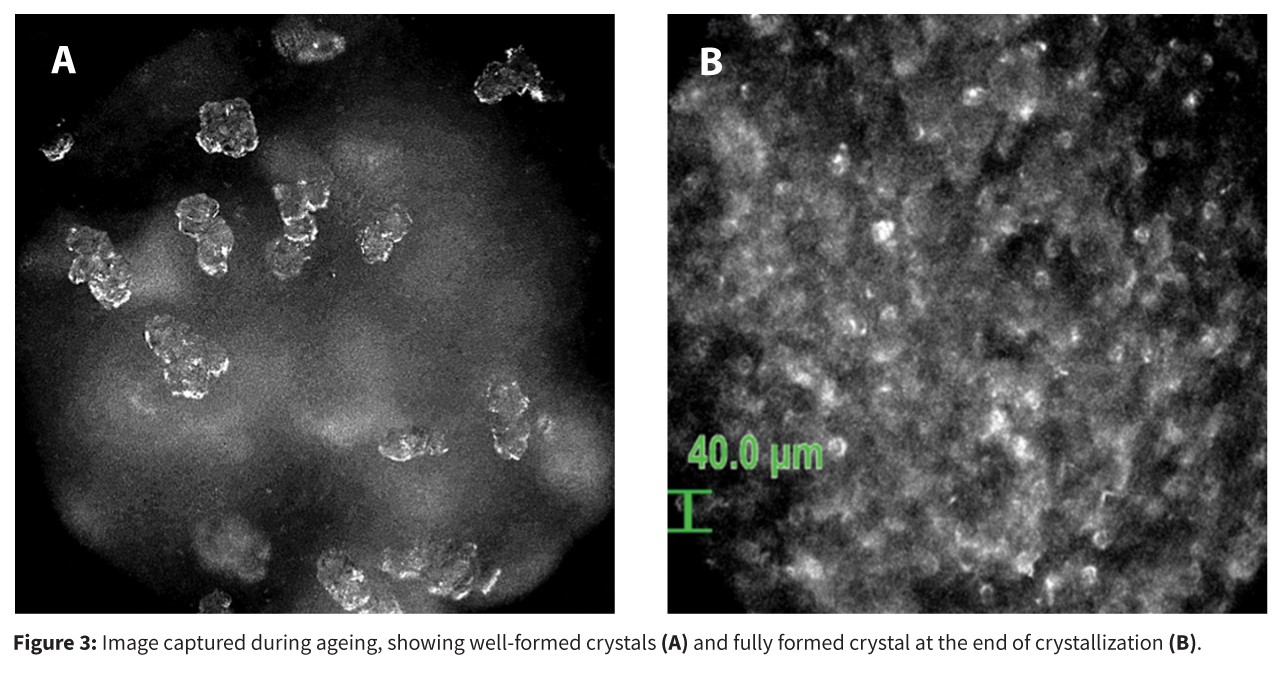
These interventions resulted in:
This case study highlights the importance of thermodynamic and kinetic control in industrial crystallization, the utility of PAT tools for real-time process monitoring, and the value of tailored solvent and seeding strategies to prevent oiling out—a phenomenon increasingly recognized as a key challenge in pharmaceutical manufacturing.
At Aragen, we leverage particle science and advanced technologies to optimize your drug substance for stability, performance, and manufacturability—delivering tailored solutions from early research to commercial scale-up. We offer:
Whether your goal is to optimize crystallization performance, solve complex scale-up issues, or reduce risk in manufacturing, Aragen brings the tools and expertise to get you there—faster and more reliably.
