
Degrader-Antibody Conjugates (DACs) are novel class of therapeutics designed for selective protein degradation inside target cells. Among these, PROTAC-based DACs stand out for their potent and selective protein degradation. The PROTAC-based DACs combine a target-specific monoclonal antibody (mAb) with a PROteolysis-TArgeting Chimeras (PROTACs) or molecular glues via a linker. This approach offers advantages over traditional PROTACs, including improved physiochemical properties, solubility, permeability, bioavailability, and target tissue specificity.
The development of PROTAC-based DACs requires careful selection of tissue target- and target-specific antigens, antibody generation, design of target protein-specific PROTAC molecules, appropriate linker selection, precise conjugation technologies and in-house purification processes. Of these steps, conjugation of the PROTAC to the antibody—typically using cysteine-based methods—followed by thorough purification, is essential for developing high-quality PROTAC-based DACs.
This whitepaper presents a case study showcasing Aragen’s expertise in PROTAC-antibody conjugation using a cysteine-based strategy, demonstrating our capability to deliver robust, high-quality PROTAC-based DACs.
Targeted protein degradation (TPD) strategies including PROTACs, LYTACs, molecular glues etc.—are redefining drug discovery by enabling the elimination, rather than merely inhibition of disease-driving proteins. PROTACs as innovative bifunctional molecules, are at the forefront, allowing therapeutic targeting of previously “undruggable” proteins by hijacking the cell’s ubiquitin-proteasome system. Since their landmark introduction in 2001 by Craig Crews and Raymond Deshaies, PROTACs have made significant advances, reaching clinical trials (Phase 1-3) against a range of challenging targets. However, most PROTACs fall outside the “beyond Rule of 5” space, limiting their solubility, permeability, and oral bioavailability.
The emergence of Antibody Drug Conjugates (ADCs) has also revolutionized drug development. Since the first USFDA approval of an ADC (Mylotarg in 2001, for AML), over 15 ADCs have received approvals for various indications. While ADCs have achieved selective and tissue-specific delivery of cytotoxic payloads, challenges such as drug resistance, off-target toxicity (due to premature payload release), and other side effects persist.
Building on the success of both the modalities, PROTAC-based DACs represent a promising evolution in TPD and targeted therapy. They integrate an antibody’s antigen-binding specificity and prolonged halflife with PROTAC-mediated protein degradation activity. This synergy enhances therapeutic index, tissue specificity, and pharmacokinetic/ pharmacodynamic (PK/PD) profiles, offering a promising platform for precision medicine across oncology, autoimmune, and neurodegenerative diseases. Successful PROTAC-based DAC development requires a careful selection of target antigens and antibodies, design of selective PROTACs, optimized linker chemistry, and robust conjugation and appropriate purification workflows.
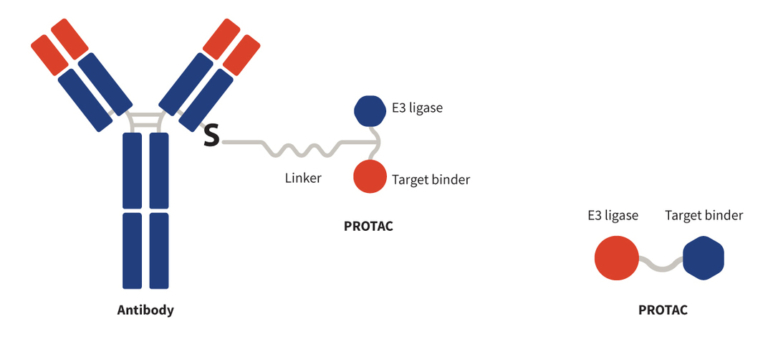
The design of PROTAC-based DAC hinges on the combination of antibody-mediated targeting of specific tissues and cell types along with the intracellular protein degradation facilitated by the degrader PROTAC payload. Each component must be meticulously engineered to maintain conjugate stability, specificity, and potency. A typical PROTAC-based DAC consists of three essential components (Figure 1):
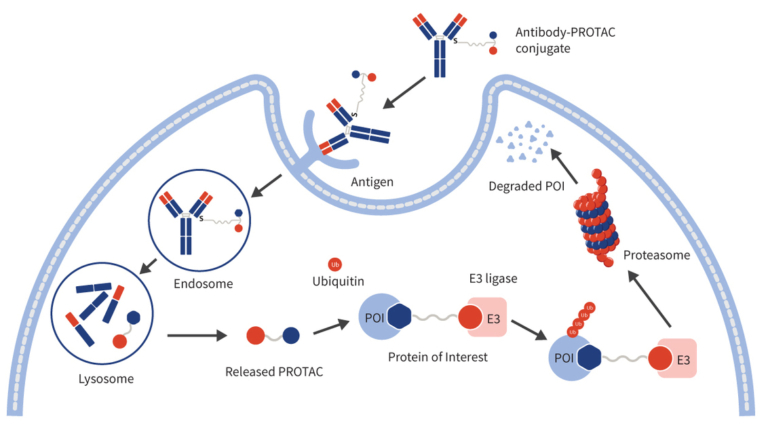
Mechanistically, the antibody component of PROTAC-based DAC docks on the overexpressing antigen of the target cells. Thereafter, PROTAC-based DAC-antigen complex gets internalized into the cell via receptor-mediated endocytosis to form an endosome. The endosome inside the cell is trafficked to lysosome where antibody and linker is cleaved leading to release of PROTAC payload in cytosol. Upon release, PROTAC forms ternary complex with target protein and E3-ubiquitin ligase, facilitating polyubiquitination and proteasomal degradation of the protein. This mechanism achieves potent intracellular protein clearance while minimizing systemic exposure and off-target toxicity (Figure 2).
Challenge
The development of PROTAC-based DACs is a complex technical challenge that lies at the intersection of chemistry, process engineering, and analytical precision. Achieving a defined and reproducible DAR in PROTAC-based DACs remains a critical challenge in bioconjugation chemistry. It requires meticulous optimization of conjugation parameters, significant resource investments, and robust purification workflows. Furthermore, implementation of suitable purification strategies is essential to eliminate free payloads, aggregates, and reaction by-products to significantly improve the product quality and therapeutic efficacy.
Aragen’s Approach
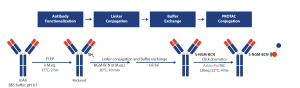
At Aragen, we utilized our expertise in protein bioconjugation to develop and characterize PROTAC-based DACs. The process includes screening of various linkers, fine-tuning of conjugation reaction conditions, and developing post-conjugation purification strategies. Our standard experimental workflow is outlined below:
Using this approach, we developed a method, where PROTAC was conjugated to mAb using cysteine-directed conjugation chemistry. The antibody was first buffered into BBS buffer, followed by reduction with 6 molar equivalents of TCEP and incubation at 37°C for 2 hours. After reduction, the temperature was lowered to 20°C, then 8 molar equivalents of a maleimide-based linker (NGM-BCN) were added and incubated at 20°C for 10 min to conjugate the linker to reduced cysteine residues. A subsequent buffer exchange using UF/DF with acetate buffer removed unconjugated linker. Then, 20 molar equivalents of azido-PROTAC (solubilized in DMF) were added and click chemistry-based conjugation was performed by incubating the mixture at 22°C for 4 hours. Figure 3 illustrates the systematic workflow implemented in this case study.
The purified PROTAC-based DAC was analyzed by SDS-PAGE, SEC-HPLC and LC-MS. The LC-MS data was used to calculate DAR by determining the number of payloads attached to heavy and light chains based on mass differences before and after conjugation. The percentage area for each species were calculated from mass spectra data (Figure 4). The following formula was used to calculate the average DAR:
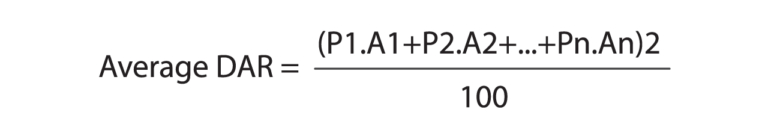
Here, “P” represents the number of payloads per chain and “A” denotes the corresponding percentage peak area, and 1, 2, …n represents different heavy and light chain species post-conjugation.
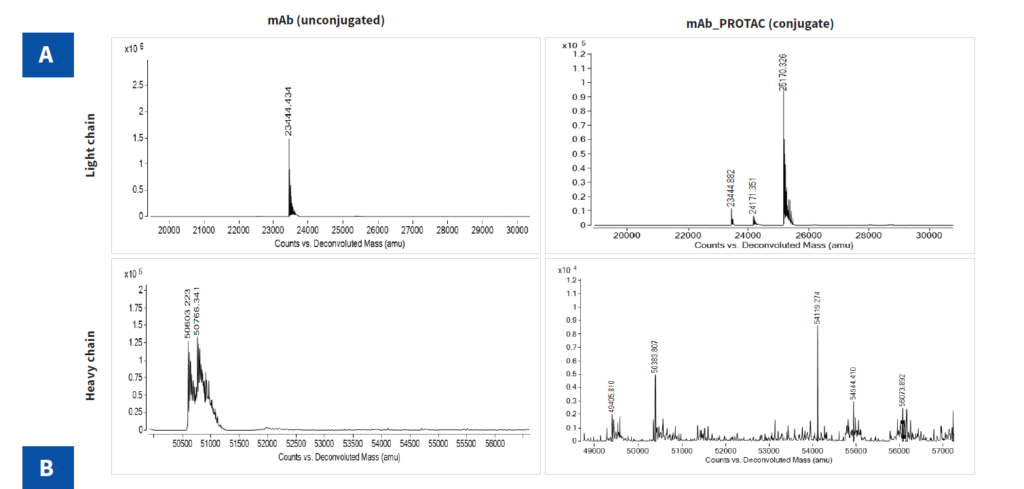
| mAb Chain | Glycan | Observed Reduced Mass (Da) | Mass difference (ΔM in Da) | No. of Payload per chain (P) | Mass Peak percentage Areas (A) | DAR | |
|---|---|---|---|---|---|---|---|
| mAb Pre-conjugation | mAb-NGM-BCN PROTAC Post-conjugation | ||||||
| Heavy chain | G0 | 50383 | 50384 | 1 | 0 | 28 | 5.07 |
| G0F | 50603 | 54119 | 3516 | 2 | 30 | ||
| GIF | 50765 | 54994 | 4229* | 2 | 12 | ||
| G2F | 50930 | 56074 | 5144 | 3 | 11 | ||
| G2F | 50930 | 56162 | 5232 | 3 | 19 | ||
| Light chain | 23444 | 23445 | 1 | 0 | 15 | ||
| 24171 | 727* | 0 | 6 | ||||
| 25170 | 1726 | 1 | 79 | ||||
We successfully optimized the cysteine-based conjugation reaction and purification process, followed by rigorous quality checks and DAR estimation. The average DAR of the resulting PROTAC-based DAC in this study was 5.07. This case study demonstrates our capability in developing high-quality degrader antibody conjugates.
PROTAC-based degrader antibody conjugates combine a PROTAC molecule with a cell surface antigen-specific antibody, representing a powerful next-generation therapeutic approach for selective protein degradation in target tissues such as neoplasms or diseased cells. Producing a high-quality PROTAC-based DAC is a complex process that requires a well-designed systematic workflow, precise conjugation chemistry, optimized purification methods, and rigorous analytical characterization. The presented case study highlights a cysteine-directed conjugation strategy and scalable DAR determination protocol for efficient development of high-quality PROTAC-based DACs.
At Aragen, we continue our DAC development capabilities by expanding our conjugation strategy portfolio. Ongoing advances in conjugation techniques and analytical methodologies will be critical to accelerating DAC production and facilitating clinical translation.
With nearly a decade of expertise in antibody engineering and protein bioconjugation, Aragen offers comprehensive end-to-end DACs development solutions:
